Chloroacetic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
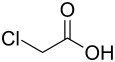
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Chloroacetic acid | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 2 H 3 ClO 2 | ||||||||||||||||||
| Brief description |
colorless solid with a pungent smell |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 94.50 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.58 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
61 ° C |
||||||||||||||||||
| boiling point |
189 ° C |
||||||||||||||||||
| Vapor pressure |
2.14 Pa (20 ° C) |
||||||||||||||||||
| pK s value |
2.87 (25 ° C) |
||||||||||||||||||
| solubility |
very good in water (4210 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−509.7 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
In chloroacetic acid (also: monochloroacetic acid ) a hydrogen atom in the methyl group of acetic acid has been replaced by a chlorine atom.
presentation
The preparation takes place by chlorination of acetic acid at 85 ° C and up to 6 bar with the addition of catalytic amounts of acetic anhydride or acetyl chloride .
properties
Chloroacetic acid forms colorless crystals with a pungent (acetic acid-like) odor that melt between 53 and 63 ° C (depending on the modification) and dissolve easily in water , but also in ethanol , diethyl ether and other organic solvents. The aqueous solution is strongly acidic, much more acidic than acetic acid.
The reason for this is the stabilization of the anion by the rather electronegative chlorine atom: It has an electron-withdrawing effect and distributes (delocalizes) the negative charge of the anion over the entire molecule and stabilizes the anion.
use
Chloroacetic acid is the starting material for carboxymethyl cellulose , mercaptoacetic acid and for pesticides , dyes and pharmaceuticals . Monochloroacetic acid is used directly to burn warts (trade name: Acetocaustin ).
The use of monochloroacetic and monobromoacetic acid as disinfectants and preservatives in the beverage industry led to the beer scandal in Bavaria in 1985 .
hazards
Chloroacetic acid and its vapors are poisonous and highly corrosive to the eyes , respiratory tract and skin . The substance is easily absorbed through the skin. If the skin comes into contact, the acid must be rinsed off immediately with water. There is a risk of poisoning which, if 5 to 10% of the body surface is wetted, or an 80% solution or more, can lead to death.
See also
Web links
Individual evidence
- ↑ Entry on CHLOROACETIC ACID in the CosIng database of the EU Commission, accessed on February 24, 2020.
- ↑ a b c d e f g Entry on chloroacetic acid in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Registration dossier on Chloroacetic acid (section Vapor pressure ) at the European Chemicals Agency (ECHA), accessed on July 27, 2016.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dissociation Constants of Organic Acids and Bases, pp. 8-42.
- ↑ Entry on Chloroacetic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-21.
- ↑ Cantonal Office for Food Inspection St.Gallen: 125 years of the Cantonal Laboratory, anniversary publication 1878 - 2003 ( Memento of January 8, 2006 in the Internet Archive ).
- ↑ Wolfgang Wagemann: Solved - New disinfectant for CIP applications . In: Brauindustrie, 2000 (11), pp. 638-640.


