Carboxymethyl celluloses
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|

|
|||||||
|
Carboxymethyl cellulose ( R = CH 2 COOH) (average degree of substitution ≈ 67%) |
|||||||
| General | |||||||
| Surname | Carboxymethyl cellulose | ||||||
| other names | |||||||
| CAS number |
|
||||||
| Monomers / partial structures | carboxymethylated cellobiose | ||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| solubility |
|
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
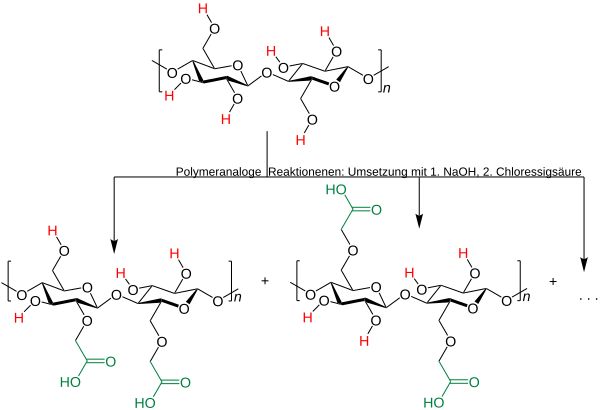
Carboxymethyl celluloses ( CMC ) are cellulose ethers , derivatives of cellulose in which some of the hydroxyl groups are linked as ethers to a carboxymethyl group (-CH 2 -COOH). The cellulose is obtained from conifers and hardwoods.
Manufacturing
Cellulose or pulp is ground and converted into the more reactive alkali cellulose with caustic soda. The alkylation of the alkali cellulose to carboxymethyl cellulose takes place with chloroacetic acid . The cellulose structure is retained.
Since not all hydroxyl groups react in this reaction, mixtures are formed with different degrees of substitution . The degree of substitution of the individual starch components within a polymer can also vary.
properties
In the acid form, carboxymethyl celluloses are insoluble in water. In contrast, they are readily soluble in basic solutions.
A water-insoluble variant of carboxymethyl cellulose produced by cross-linking is croscarmellose sodium .
Possible impact on health
Carboxymethyl celluloses could weaken the mucus barrier in the intestines of mammals. The mucus barrier isolates the intestinal contents from the intestinal wall. In mice without the IL-10 gene, the substance in the feed caused bacterial overgrowth of the intestinal mucosa and signs of inflammation similar to Crohn's disease . Particularly sensitive mice fed carboxymethyl cellulose developed more frequent and more severe colon inflammations. Such effects have not yet been demonstrated in humans.
application
The most important application for carboxymethyl cellulose is in the textile industry . Their good solubility and film-forming properties are used for finishing yarns or filaments.
In addition, carboxymethyl cellulose is used as a detergent additive , binder , thickener, paper sizing agent, protective colloid and in drilling fluids for oil wells.
It is approved in the EU as a food additive with the number E 466 , in the USA by 21CFR 182.1745. In addition to the many technical applications, cellulose derivatives also play an important role in everyday life. They are used in the food sector and many cosmetic products as well as pharmaceutical applications. CMC improves the consistency of many foods, such as ice cream (reduction of ice crystal formation), mayonnaise , sauces, fruit masses, jelly . In the case of baked goods, the starch retrogradation is delayed and with it staling, in the case of dry products the stability is increased and the rehydration ability improved.
In pharmacy, they are used as tablet disintegrants with a mass fraction of 1% and to form a hydrocolloid matrix (mass concentration 5–10%).
Economical meaning
The market for cellulose derivatives ( cellulose ethers and cellulose esters ) is estimated at over 100,000 t in Germany. Of these, carboxymethyl celluloses are the most important market in terms of volume. In 1999, 2200 t of CMC were used in detergents in Germany alone. Since cellulose ether products are made from renewable raw materials , they are becoming increasingly important, e.g. B. as a textile auxiliary for environmentally friendly processes in the textile industry.
Individual evidence
- ↑ Entry on CELLULOSE GUM in the CosIng database of the EU Commission, accessed on August 6, 2020.
- ↑ Entry on E 466: Sodium carboxy methyl cellulose, cellulose gum in the European database for food additives, accessed on August 6, 2020.
- ↑ a b c Entry on carboxymethyl cellulose. In: Römpp Online . Georg Thieme Verlag, accessed on June 11, 2013.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Alexander Swidsinski, Victoria Ung, Beate C. Sydora, Vera Loening-Baucke, Yvonne Doerffel: Bacterial Overgrowth and Inflammation of Small Intestine After Carboxymethylcellulose Ingestion in Genetically Susceptible Mice . In: Inflammatory Bowel Diseases . tape 15 , no. 3 , p. 359-364 , doi : 10.1002 / ibd.20763 , PMID 18844217 ( wkhealth.com [accessed March 3, 2017]).
- ^ Sara Reardon: Food preservatives linked to obesity and gut disease. In: Nature , February 25, 2015, doi : 10.1038 / nature.2015.16984 .
- ↑ Oliver Türk: Material use of renewable raw materials . 1st edition. Springer Vieweg, Wiesbaden 2014, ISBN 978-3-8348-1763-1 , p. 208-209 .
- ↑ Analysis and characterization according to USP
- ↑ foodnews: Cellulose derivatives .
- ^ Rudolf Voigt: Pharmaceutical Technology. 11th edition, 2010, Deutscher Apotheker Verlag, ISBN 978-376-925003-9 .
- ^ FNR [specialist agency for renewable raw materials] (2006): Market analysis of renewable raw materials; Gülzow.
- ^ Industrial Association for Body Care and Detergent (IKW), September 2000.