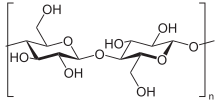
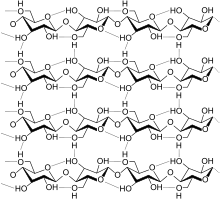
cellulose
As pulp (English chemical pulp or short pulp ) is known in the chemical digestion resulting from plant fibers fibrous mass consisting mainly of cellulose consists. Besides wood pulp , pulp is an important raw material in paper production . 90% of the pulp produced worldwide is made from wood. The use of straw , bagasse , kenaf or bamboo as raw materials is falling significantly worldwide.
The production of cellulose takes place regionally differently from industrial waste wood or plantation wood . In Europe and North America, for example, saw residue (mostly softwood ) is used. In South America, South Africa and Australia, hardwood from forest plantations (fast-growing eucalyptus ) is often used. The wood is first debarked and then processed into chipped wood (wood chips). The wood is then chemically digested:
- The alkaline sulphate process , with which over 95% of world production is made, is predominant . Both hardwood and softwood can be used.
- There is also the acid sulphite process . Only 2% to 3% are produced using this process. The breakdown of wood with a high resin content , such as pine , is rare here. Mostly spruce , beech or eucalyptus wood is used. The sulphite process has a stable market niche in the production of viscose pulp .
A qualitative distinction is made between softwood and hardwood pulp. Softwood sulphate pulp has long, firm fibers averaging 3.3 mm in length, which gives paper a reinforcement . Hardwood pulp has shorter fibers about 1 mm in length. It is suitable for the production of smooth papers (writing, copier paper ) or tissue (hygiene paper).
history
In the beginning, paper was made exclusively from rags . In the 19th century, the increasing need for paper led to the development of pulp and wood pulp.
The digestion of wood with caustic soda (NaOH) was developed in 1851 by H. Burgess and C. Watt in England and introduced in the USA in 1854. The high demand for sodium hydroxide made evaporation, incineration and re- causticizing too expensive. C. Dahl in Danzig tried to add sodium sulfate (Na 2 SO 4 ) directly into the recovery system. The sodium sulfite (Na 2 SO 3 ) produced by reduction significantly improved the fiber quality. In 1884 Dahl received a patent for the process modification. The alkaline conditions resulted in very strong fibers which, however, were colored dark brown. For a long time, they were only used for the production of unbleached paper products (paper sacks, cardboard boxes ).
In 1867 the sulphite digestion was patented for BC Tilghman in the USA (USP 70,485). This procedure uses a solution of calcium bisulfite (Ca (HSO 3 ) 2 ) and free sulfur dioxide (SO 2 ). The first large-scale plant was built in Sweden in 1874 under CD Ekman, it used small, rotating cookers and magnesium bisulfite (Mg (HSO 3 ) 2 ). In Germany, the Tilghman von Mitscherlich sulfite process was introduced in 1875. Mitscherlich used lying stoves, low temperatures of 110 ° C and very long rotation times. The acidic sulphite boiling according to Mitscherlich leads to comparatively light fibers. When using wood chips from well debarked wood, a degree of whiteness of almost 70% ISO can be achieved. Chlorine bleaching with chlorinated lime was used to lighten the fibers further .
raw material
Source:
| Wood species | Eucalyptus globulus | Eucalyptus grandis | birch | Pine trees | Spruce trees | beech | Acacia | Poplars |
|---|---|---|---|---|---|---|---|---|
| Distribution area | Iberian Peninsula | South America | Northern hemisphere | Northern hemisphere | Northern hemisphere | Northern hemisphere | India , Indonesia | |
| Yield [m 3 / ha / year] |
|
3-8 | 2-10 | 4-10 | 2-9 | 15-25 | ||
| Service life [years] |
|
25-45 | 75-110 | 60-80 | 100-140 | 6-12 | ||
| Yield [m 3 wood / t pulp] |
2.8-2.9 | 3.8-4.0 | 4.5 | 5 | 3 | 4.9 | ||
Manufacturing
Wood chips that are as evenly shaped as possible are required for pulp production . The mechanical comminution improves the uniform impregnation of the wood with the digestion solution. Very coarse wood chips are not completely impregnated, which leads to undigested residues. Too fine wood chips have the disadvantage of a strong mechanical shortening of the fibers. Sawmill waste (chippings) or chipped wood are hardly suitable. Wood mainly consists of lignocellulose . During the digestion, the incrustations of the lignin within the cellulose matrix are split up and dissolved, while the structure of the cellulose is largely retained.
Sulfate process
- (see main article sulphate process )
The sulphate process (also called sulphate digestion or process) is also called the Kraft process because of the stronger fibers . It is named after the “make-up chemical” of the process, Glauber's salt (sodium sulphate, Na 2 SO 4 ). The active substances are caustic soda and sodium sulfide. The digestion takes place mainly in continuous digesters. Modifications to the original batch process are also applied. The wood chips are impregnated with the cooking liquor and fed into the standing reactors from above . At a temperature of up to 170 ° C, wood components such as hemicelluloses and lignins are dissolved in an alkaline manner . The lignin is depolymerized by nucleophilic ether cleavage of the sulfite ions (SO 3 2− ) . Degradation of the cellulose fibers is undesirable, which is why the digestion process is stopped before all of the lignin has dissolved. Unbleached cellulose therefore still contains around 2% to 3% lignin . Up to 1.3 million tons of pulp are produced annually in a production line of a pulp production plant.
In Germany, two pulp mills operate using the sulphate process: Zellstoff- und Papierfabrik Rosenthal GmbH in Blankenstein / Thuringia with an annual capacity of 360,000 tons, and Zellstoff Stendal GmbH near Arneburg / Saxony-Anhalt, which has an annual capacity of 660,000 tons.
Sulfite process
- (see main article sulphite process )
The sulfur dioxide required for the sulphite process (also called sulphite digestion or process) was previously produced by direct burning of sulfur or roasting of sulphidic ore and passed through a wooden tower with limestone. By sprinkling with cold water, the required calcium bisulfite solution was formed with additional free sulfur dioxide. Liquid sulfur dioxide is used today. The acid sulphite process breaks the bond between lignin and cellulose by sulphonation and ether cleavage of the lignin. The low pH value damages the structure of the cellulose chains on the acetal bridges between the sugar molecules. The crystalline area of the cellulose remains stable. The fiber strength of sulphite pulp is worse than that of sulphate pulp. The hydrophilic , sulfonated lignin is readily soluble in water and can be processed into lignosulfonates . These are used as auxiliaries for disperse dyes .
The manufacture of paper almost always requires pulp bleaching , which dissolves lignin residues through oxidation . During the sulphite process, hemicelluloses are also dissolved by the alkaline bleach.
The yields per dry mass of wood are 40% to 45% for the sulphate process and 45% to 50% for the sulphite process. The wastewater pollution correlates directly with the amount of dissolved substance.
Other procedures
Other methods, such as the soda - (Na 2 CO 3 · 10H 2 O) and the soda / anthraquinone process ( Soda / AQ process ), wherein the anthraquinone as a catalyst for a better delignification serves play no role industrially. The alkaline sulphite digestion with the addition of anthraquinone and methanol (ASAM process) or the natural pulping process that works with performic acid and the similar Acetocell process with peroxyacetic acid digestion should also be mentioned. These processes have been tested in pilot plants, but are not economical.
The Organocell process with caustic soda and methanol (CH 3 OH) also proved to be uncompetitive. A large-scale plant based on this principle in Kelheim was put into operation in 1992 with considerable government funding, but was shut down in 1993 due to quality problems.
The experimental digestion using formic and acetic acid is an environmentally friendly approach.
Chemical recovery
In the classic Mitscherlich process, the waste liquor from the calcium sulphite digestion was disposed of via the receiving water. Thus, half of the wood mass used polluted the wastewater and water bodies. Modern pulp plants recover the pulping chemicals. Because of the formation of calcium sulphate deposits ( gypsum ), only the acidic magnesium sulphite process is used in Central Europe - with a few exceptions. After “boiling” the pulp is washed and the waste water is evaporated. The acidic " black liquor " is obtained. When they are "burned", magnesium sulfite is thermally split into magnesium oxide (MgO) and sulfur dioxide. MgO is deposited on electrostatic filters and converted back into magnesium bisulfite (Mg (HSO 3 ) 2 ) in Venturi reactors with the SO 2 contained in the flue gas .
When the " black liquor " (black does not refer to the color, but rather the low purity) is burned with reduced air intake, a melt of sodium carbonate (Na 2 CO 3 ) and sodium sulfide (Na 2 S) is formed. " Green liquor " is obtained by dissolving in water . The sodium carbonate is causticized with calcium hydroxide (slaked lime, Ca (OH) 2 ) to form sodium hydroxide solution . Filtration creates the " white liquor ". This is used again in the process. The filtered calcium carbonate is burned again to calcium oxide (quick lime, CaO) in a rotary kiln .
This recovery not only relieves the receiving water, but the required process energy is also obtained from the waste liquor. Modern systems generate a surplus of electrical energy. The excess steam can be used for paper drying in an integrated pulp and paper production.
Small pulp plants usually do not have a recovery plant. The severe environmental pollution caused by the high content of lignin and hemicelluloses in the wastewater is increasingly leading to shutdown. In China alone, over 10,000 straw pulp mills with an annual output of less than 5,000 tons have been closed by the government in the past ten years due to the pollution of rivers and lakes. The effort for the recovery of the chemicals is too high in small plants.
Pulp quality
The strength of the cellulose fiber is crucial for the production of some types of paper. Pulp to improve paper strength is known as reinforcement pulp. It has long and thin fibers. Softwood from regions with a cool winter but a warm summer has these properties. This pulp is used in the production of wood-containing paper (magazine, catalog paper). In regions with a long growing season , softwood fibers are produced that are both long and relatively thick. These coarse fibers are ideal for making solid paper bags or for coffee filters .
The short fibers of the hardwoods enable a smooth, even paper surface. High -quality paper can only be made from hardwood pulp on paper machines . Mixtures of long and shorter fibers are required for older machines.
The pulp manufacturing process has a significant impact on fiber quality. Loss of quality is caused by shortening the fibers of the wood chips, too intensive digestion or during pulp bleaching. Quality values of mechanical strength tests are breaking length or tear strength . In addition, there is the relationship between the degree of polymerisation of the cellulose and the fiber strength. The analysis of the viscosity of a dissolved fiber sample provides information on the degree of fiber damage.
Pulp is produced in different qualities. Northern bleached long fiber sulphate pulp, usually called NBSK pulp or NBSK (abbreviation for Northern bleached softwood kraft ), is used in the manufacture of various types of paper. Cellulose wadding ( fluff pulp ) is made from softwood, has a high water-binding capacity and is mainly used for hygiene products such as diapers . Chemical pulp ( dissolving pulp ) has a low color, a uniform molar mass distribution and is not used for paper production, but for chemical processing; hardwoods with a high cellulose content are used to increase the quality of the end product.
Pulp trade

Green: bleached with elemental chlorine (Cl 2 )
Blue: ECF ( elemental chlorine free ), d. H. bleached with chlorine dioxide or chlorite
Gray: TCF ( totally chlorine free ), d. H. bleached with ozone or hydrogen peroxide
Pulp is mainly traded bleached. For 2004 the world production of bleached pulp was estimated at 80 million tons. Today, ECF (elemental chlorine-free) bleached grades with a whiteness of 88% to 90% ISO for softwood pulp and over 90% ISO for hardwood pulp are leading. Bright white hardwood pulps with a whiteness of over 92% ISO are used to produce photo paper .
Another quality feature is the stability against yellowing. This can be low with TCF (total chlorine free) bleached hardwood pulp.
use
- paper
- carton
- Paper filters (mostly filter bags in the household )
- Cotton wool and bandages
- Paper handkerchiefs
- Hygiene products such as tampons , sanitary napkins , pant diapers and aids for urinary or fecal incontinence
- Reinforcement of plaster of paris or cement as a composite material
- Pulp composites
Chemical pulp products :
- Cellulose derivatives such as cellulose esters or ethers
- Cellulose regenerates such as viscose , lyocell , modal, copper silk , foils
Environmental aspects
→ see paper: environmental aspects and recycling
See also
Web links
Individual evidence
- ↑ a b c d e f H. Sixta, Handbook of Pulp, 2006, ISBN 3-527-30999-3 .
- ↑ ENCE : Sustainable Forest management and Eucalyptus (PDF), 2009, p. 32.
- ↑ Requirement of dry wood in m 3 for the production of 1 ton of air-dry pulp
- ^ Paper Lexicon, Deutscher Betriebswirte-Verlag, Gernsbach 1999, Vol. 1, 88, ISBN 3-88640-080-8 .
- ^ Paper Lexicon, Deutscher Betriebswirte-Verlag, Gernsbach 1999, Vol. 1, 41, ISBN 3-88640-080-8 .
- ^ Paper Lexicon, Deutscher Betriebswirte-Verlag, Gernsbach 1999, Vol. 2, 379, ISBN 3-88640-080-8 .
- ↑ Jan Beringer: Pulp from wheat straw: Obtaining by digestion processes with formic and acetic acid as well as studies on the pulp structure and suitability as paper and chemical pulp. In: Logos Verlag Berlin, 2005, ISBN 978-3-8325-0797-8 .
- ^ H. Holik, Handbook of Paper and Board, VCH-Wiley, Weinheim 2006 ISBN 3-527-30997-7 .
- ↑ Christopher J. Biermann: 3 . In: Handbook of Pulping and Papermaking , 2nd edition 1996, ISBN 978-0-12-097362-0 , pp. 72-73.
- ↑ FC Aldred: Pulping Quality in Plantation Grown species . In: The Commonwealth Forestry Review . 46, No. 4, December 1967, pp. 270-277.
- ↑ Keyword "bleaching". In: Ullmann's Encyclopedia of Industrial Chemistry 7th Edition 2007 Wiley-VCH Verlag GmbH & Co. KGaA online edition.