Degree of substitution
| Degree of substitution | Primary | Secondary | Tertiary | quaternary |
|---|---|---|---|---|
| C atom of organic compounds |

|

|

|

|
| Alcohols |

|
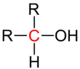
|

|
not existent |
| Amines |

|

|

|

|
| Phosphines |

|

|
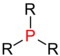
|
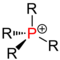
|
| Amides (not IUPAC compliant) |

|
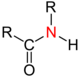
|

|
not existent |
In organic chemistry, the degree of substitution describes the number of alkyl or aryl groups on a particular atom . The IUPAC defines the degree of substitution as the number of hydrogen atoms that have been replaced by hydrocarbons on an atom . In contrast to the degree of substitution, the bond order also includes the number of other groups.
properties
The alkyl or aryl groups are referred to as substituents on an atom. An atom with such a substituent is primary , one with two substituents is secondary , one with three substituents is tertiary, and one with four substituents is quaternary . The inductive effect of the respective atom increases with the degree of substitution with aryl or alkyl groups . In atoms with a lone pair of electrons such as nitrogen , the basicity increases with the degree of substitution.
The term is also applied to molecules , e.g. B. the degree of substitution of repeating units of polymers . The number is described as single, double, triple, etc.
Applications
In the case of alcohols , the degree of substitution was determined using the Lucas sample .
Individual evidence
- ↑ John E. McMurry: Fundamentals of Organic Chemistry. Cengage Learning, 2010, ISBN 978-1-439-04971-6 , p. 405.
- ^ GP Moss, PAS Smith, D. Tavernier: Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). In: Pure and Applied Chemistry. 67, 1995, doi : 10.1351 / pac199567081307 .
- ^ Francis A. Carey: Advanced Organic Chemistry. Springer Science & Business Media, 2000, ISBN 978-0-306-46243-6 , p. 298.