Lucas sample
The Lucas test (also known as the Lucas test ) is a detection reaction in organic chemistry to differentiate between primary , secondary and tertiary alcohols. Depending on the position of the hydroxyl group in the alcohol molecule , alcohols react with nucleophilic substitution at different speeds or not at all. Since the alcohol must completely dissolve in the added Lucas reagent , the test is limited to alcohols with fewer than six carbon atoms.
The reagent
The Lucas reagent - named after Howard J. Lucas - is a solution of zinc chloride in concentrated hydrochloric acid ; The hydrochloric acid is a reactive component and zinc chloride serves as a catalyst. This mixture had already been used by Norris and Taylor for other purposes before the publication of his test, as Lucas himself writes.
execution
To carry out the Lucas test, a few drops of the alcohol to be tested are placed in the Lucas reagent and shaken. The test is positive if a milky-cloudy liquid forms. This happens at different speeds, depending on the position of the hydroxyl group:
- Primary alcohols: reaction only when the sample is heated
- Examples: ethanol or 1-propanol
- Secondary alcohols: reaction within a short time (about five minutes)
- Examples: 2-propanol or 2-butanol
- Tertiary alcohols: instantaneous reaction
- Example: 2-methyl-2-propanol .
The reaction time is longer, the smaller the ratio of reagent to alcohol and the lower the temperature.
Explanation
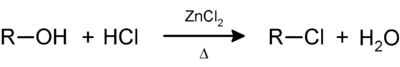
Low molecular weight alcohols dissolve very well in water and also in the Lucas reagent. Depending on the position of the hydroxyl group, the alcohols react with the reagent in that the OH group is substituted by a chlorine atom. The inert primary alcohols do not react noticeably and the solution remains clear. Secondary alcohols react in a few minutes, and the resulting chlorinated hydrocarbon initially clouds the solution and then separates out as a water-insoluble phase. The much more reactive tertiary alcohols instantly show turbidity and phase separation:
Exceptions
An exception is allyl alcohol , in which the reaction - like the tertiary alcohols - occurs immediately. In addition, the resulting allyl chloride is soluble in the Lucas reagent , so that the necessary cloudiness does not occur.
Phenols give a negative result, as neither an S N 2 reaction - this requires a nucleophilic attack from the rear, i.e. from the inside of the ring - nor an S N 1 reaction - a phenyl cation is not formed under these conditions - is possible.
Individual evidence
- ↑ a b Paula Y. Bruice and Thomas Lazar, Organic Chemistry (2007), p 502 ISBN 978-3-8273-7190-4
- ↑ Norris and Taylor, J. Am. Chem. Soc 46 , 753 (1924)
- ↑ HJ Lucas, J. Am. Chem. Soc 52 , 802 (1930)
literature
- Heinz GO Becker: Organikum : basic organic-chemical internship . 23rd edition. Wiley-VCH, Weinheim 2009, ISBN 978-3-527-32292-3 , pp. 700 (edited by Rainer Beckert).
- Differentiation between primary, secondary and tertiary alcohols . Chemistry teacher training course - Dr. Tear at the Philipps University of Marburg. (Accessed January 27, 2009)