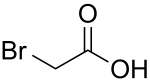
Bromoacetic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Bromoacetic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 2 H 3 BrO 2 | |||||||||||||||||||||
| Brief description |
white to yellowish solid with a pungent odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 138.95 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.93 g cm −3 |
|||||||||||||||||||||
| Melting point |
46-49 ° C |
|||||||||||||||||||||
| boiling point |
208 ° C |
|||||||||||||||||||||
| Vapor pressure |
15.8 Pa (25 ° C) |
|||||||||||||||||||||
| pK s value |
2.89 (25 ° C) |
|||||||||||||||||||||
| solubility |
soluble in water (93.8 g l −1 ) |
|||||||||||||||||||||
| Refractive index |
1.4804 (50 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Bromoacetic acid (also: monobromoacetic acid ) is a derivative of acetic acid in which one hydrogen atom of the methyl group is replaced by a bromine atom . Their salts are called (mono-) bromoacetates .
presentation
It is represented by reacting chloroacetic acid in a nucleophilic substitution reaction with potassium bromide or hydrogen bromide in the presence of aluminum chloride .
In a Hell-Volhard-Zelinsky reaction , the compound can be produced directly by reacting acetic acid with bromine in the presence of phosphorus tribromide .
properties
Bromoacetic acid forms colorless crystals with a pungent odor, which melt between 46 and 49 ° C and easily dissolve in water , ethanol , diethyl ether and other organic solvents. The aqueous solution reacts strongly acidic, significantly more acidic than acetic acid. The reason for this is the stabilization of the anion by the rather electronegative bromine atom: It has an electron-withdrawing effect and distributes (delocalizes) the negative charge of the anion over the entire molecule. The anion is therefore formed more easily than the corresponding anion of acetic acid. In aqueous solution the bromoacetic acid forms with water with the formation of oxonium ions and bromoacetate anions.
use
Bromoacetic acid is the starting material for various syntheses, for example for pesticides or pharmaceuticals .
Bromoacetic acid was used directly as a preservative for food. However, this application is now prohibited in most countries. Bromoacetic acid is strongly alkylating and has an inhibitory effect on enzymes with SH , OH or NH 2 groups in the active center. It used to be found mainly in sweet French wines. In the 1980s there was a scandal in the Federal Republic of Germany because of the banned use of bromoacetic acid as a preservative in beer by the Schäff brewery in Treuchtlingen .
Volatile esters of bromoacetic acid are used as tear gas. They are irritating to the respiratory system and eyes and can be harmful. In higher concentrations, they are corrosive and can cause permanent damage to the eyes. They must not be used by children and are therefore forbidden as joke articles (see Consumer Goods Ordinance ).
safety instructions
Bromoacetic acid and its solutions are highly corrosive and poisonous.
See also
Individual evidence
- ↑ a b c d e f g h Data sheet bromoacetic acid (PDF) from Merck , accessed on January 22, 2011.
- ↑ R. Nitzsche, R. Hildebrandt, R. Mitzner, G. Roebisch: monthly books for chemistry . 119, 1988, pp. 495-504.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-56.
- ↑ a b Entry on bromoacetic acid in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Entry on Bromoacetic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b D. Yoffe, R. Frim, SD Ukeles, MJ Dagani, HJ Barda, TJ Benya, DC Sanders: Bromine Compounds. In: Ullmann's Encyclopedia of Technical Chemistry . Wiley-VCH Verlag, Weinheim 2013, doi : 10.1002 / 14356007.a04_405.pub2 .
- ^ Robert Ebermann: Textbook food chemistry and nutrition. Springer-Verlag, Vienna 2008, ISBN 978-3-211-49348-9 , p. 590 ( limited preview in the Google book search).
- ↑ Birgit Speckle: Dispute about beer in Bavaria. Waxmann Verlag, ISBN 978-3-8309-5919-9 , p. 120 ( limited preview in the Google book search).




