Potassium bromide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
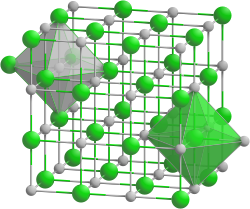
|
||||||||||||||||
| __ K + __ Br - | ||||||||||||||||
| Crystal system |
cubic |
|||||||||||||||
| Space group |
Fm 3 m (No. 225) |
|||||||||||||||
| Coordination numbers |
K [6], Br [6] |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Potassium bromide | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | KBr | |||||||||||||||
| Brief description |
colorless, hygroscopic crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 119.01 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.75 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
732 ° C |
|||||||||||||||
| boiling point |
1435 ° C |
|||||||||||||||
| Vapor pressure |
1.3 h Pa (795 ° C) |
|||||||||||||||
| solubility |
easily in water (650 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.5598 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−392 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Potassium bromide is the potassium - salt of hydrobromic forms, the colorless crystal dice even better than potassium chloride are soluble in water.
presentation
The classic method for the production of KBr takes place from the reaction between potassium carbonate and iron (II, III) bromide. The Fe 3 Br 8 is produced beforehand from scrap iron with excess bromine and a layer of water:
In the laboratory, for example, potassium bromide can be produced by reacting potassium hydroxide solution with bromine in an ammoniacal solution. This also creates water and nitrogen :
The bromination of potash also provides potassium bromide, the less soluble potassium bromate is deposited:
The production from the elements has no technical meaning:
The equations of the ion formation reaction are:
- Partial equations:
- Overall equation:
properties
Potassium bromide behaves like a cold liquid under pressure and appears transparent in IR light. It does not form hydrates .
The standard enthalpy of formation of potassium bromide is Δ H f 0 = −392 kJ / mol.
The salt is very soluble in water. The solubility increases with increasing temperature.
solubility in water temperature in ° C −10 0 20th 40 60 80 100 120 solubility in g / 100 g H 2 O 48.8 54.0 65.6 76.1 85.9 95.3 105 115
Potassium contains 0.0118% of the isotope 40 K, this provides 10242 Bq per kilogram KBr, of which 89.28% is beta radiation and 10.72% is gamma radiation with 1.46083 MeV.
use
Potassium bromide is used to produce silver bromide emulsions on films and plates for photographic films. In photographic developers, it counteracts fogging and delays development.
Because of the high optical transparency for electromagnetic waves in the range from 0.23 to 26.0 μm, single crystals of KBr are used for the production of optical components, such as e.g. B. Lenses and prisms, used for infrared optics. Since it also flows slightly cold (see above), solids for infrared spectroscopy are embedded in it, so-called potassium bromide pellets.
Potassium bromide was also used as the mid-19th century medicines for the treatment of seizures and as a sedative used. It is thus the oldest anti-epileptic . At that time z. Sometimes very high doses were used, which led to chronic bromine poisoning. This so-called bromism was characterized by excessive sedation , dizziness, headaches, lack of concentration, loss of memory, hallucinations, or rhinitis. Skin symptoms such as bromine acne or bromoderm with painful, purulent skin lumps (mostly on the limbs ) also often appeared . In the first half of the 20th century, the bromine preparations were replaced by the barbiturates .
Even today, potassium bromide is used as Dibro-Be mono for the treatment of a special form of epilepsy, the early childhood grand mal epilepsy. Bromism is rare now that lower doses are used. Today it is no longer used as a sedative.
Individual evidence
- ↑ a b c d e f g Entry on potassium bromide in the GESTIS substance database of the IFA , accessed on February 17, 2017(JavaScript required) .
- ↑ a b Data sheet potassium bromide (PDF) from Merck , accessed on January 19, 2011.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Index of Refraction of Inorganic Crystals, pp. 10-246.
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. Walter de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 (Reading sample: Part A - Basics of the chemistry of hydrogen. Google book search ).
- ↑ Chemdat .
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 1170.
- ↑ a b Yoffe, D .; Frim, R .; Ukeles, SD; Dagani, MJ; Barda, HJ; Benya, TJ; Sanders, DC: Bromine Compounds , in: Ullmanns Enzyklopädie der Technischen Chemie , Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2013; doi : 10.1002 / 14356007.a04_405.pub2 .
- ^ Locock C. Discussion of a paper by EH Sieveking: Meeting of the Royal Medical and Surgical Society of London. Lancet. 1857; 1: 527.
- ↑ Hans Bangen: History of the drug therapy of schizophrenia. Berlin 1992, ISBN 3-927408-82-4 . P. 24








