Cesium bromide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
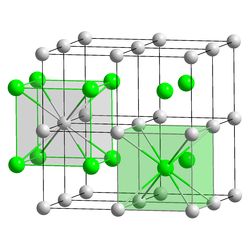
| __ Cs + __ Br - | ||||||||||||||||
| Crystal system |
cubic |
|||||||||||||||
| Space group |
Pm 3 m (No. 221) |
|||||||||||||||
| Lattice parameters |
a = 4.2953 Å |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Cesium bromide | |||||||||||||||
| Ratio formula | CsBr | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 212.809 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
4.44 g cm −3 |
|||||||||||||||
| Melting point |
636 ° C |
|||||||||||||||
| boiling point |
1300 ° C |
|||||||||||||||
| solubility |
1230 g · l −1 in water at 25 ° C, good in ethanol , insoluble in acetone |
|||||||||||||||
| Refractive index |
1.6974 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−406 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Cesium bromide is the cesium salt of hydrobromic acid .
presentation
Cesium bromide can be produced from cesium hydroxide or cesium carbonate by a salt formation reaction with hydrobromic acid :
properties
Cesium bromide forms colorless, cubic crystals and is readily soluble in water. It has a red-violet flame color . It is also soluble in lower alcohols . Like cesium chloride and cesium iodide, it crystallizes in the cubic cesium chloride structure in the space group Pm 3 m (space group no. 221) with the lattice parameter a = 4.2953 Å .
use
Windows and prisms for IR and FIR spectroscopy and for scintillation counters are made from cesium bromide crystals .
Individual evidence
- ↑ a b data sheet cesium bromide from Sigma-Aldrich , accessed on October 12, 2016 ( PDF ).
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1281.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Inorganic Compounds, pp. 4-57.
- ^ A b c G. WA Milne: Gardner's Commercially Important Chemicals: Definitions, Trade Names, and ... John Wiley & Sons, 2005, ISBN 978-0-471-73518-2 , pp. 122 ( limited preview in Google Book search).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Index of Refraction of Inorganic Crystals, pp. 10-246.
- ↑ a b data sheet cesium bromide from Sigma-Aldrich , accessed on March 15, 2011 ( PDF ).
- ↑ a b Korth crystals: Cesium bromide , accessed on October 12, 2010.


