Cesium hydroxide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ Cs + __ OH - | |||||||||||||||||||
| Crystal system |
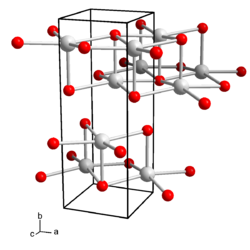
orthorhombic |
||||||||||||||||||
| Space group |
Cmcm (No. 63) |
||||||||||||||||||
| Lattice parameters |
a = 4.35 Å , b = 11.99 Å and c = 4.516 Å |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Cesium hydroxide | ||||||||||||||||||
| Ratio formula | CsOH | ||||||||||||||||||
| Brief description |
white to yellow hygroscopic crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 149.92 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
3.68 g cm −3 |
||||||||||||||||||
| Melting point |
272.3 ° C |
||||||||||||||||||
| solubility |
very light in water (3000 g l −1 at 30 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
Switzerland: 2 mg m −3 (measured as inhalable dust ) |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Cesium hydroxide , CsOH, is the hydroxide of cesium . It is one of the strongest bases in aqueous solution .
Extraction and presentation
Cesium hydroxide is formed in addition to hydrogen in the extremely violent reaction (risk of explosion) of water with cesium :
Another possibility for synthesis is the reaction of cesium oxide with water.
Violent reactions can occur during mixing.
Analogous to rubidium hydroxide , cesium hydroxide is also accessible via the sulfate :
The resulting barium sulfate precipitates quantitatively and after filtration the cesium hydroxide solution can be carefully concentrated over potassium hydroxide in a platinum dish until the salt separates out. This is then slowly heated to 300 ° C in a silver boat in a stream of dry hydrogen .
properties
Aqueous solutions of cesium hydroxide have a strongly alkaline reaction and etch glass. Cesium hydroxide crystallizes orthorhombically , space group Cmcm (space group no. 63) , with the lattice parameters a = 4.35 Å , b = 11.99 Å and c = 4.516 Å. In the crystal structure, each cesium cation is coordinated by five hydroxide anions, the anions in turn coordinate five cesium ions, resulting in a layered structure. There are also hydrates of cesium hydroxide known. The monohydrate crystallizes tetragonally, space group I 4 1 / amd (No. 141) . There are also hexagonal observed forms of the monohydrate. The dihydrate, Cs (OH) · 2 H 2 O crystallizes orthorhombically , space group Pca 2 1 (No. 29) . The trihydrate is monoclinic with the space group P 2 1 / n (No. 14, position 2) .
use
Cesium hydroxide is a high-quality base for the catalytic alkynylation of aldehydes and ketones .
Cesium hydroxide is used as an electrolyte in galvanic cells .
Individual evidence
- ↑ a b c d e Entry on cesium hydroxide in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Inorganic Compounds, pp. 4-57.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for cesium hydroxide ), accessed on March 4, 2020.
- ↑ Georg Brauer: Rubidium and Cesium hydroxide . In: Handbook of Preparative Inorganic Chemistry . Ferdinand Enke Verlag, Stuttgart 1954, p. 742 .
- ↑ H. Jacobs, B. Harbrecht: A new method of presentation for cesium hydroxide. In: Journal of Nature Research B . 36, 1981, pp. 270-271 ( online ).
- ↑ R. Černy, V. Favre-Nicolin, B. Bertheville: A tetragonal polymorph of cesium hydroxide monohydrate, CsOH · H 2 O, from X-ray powder data. In: Acta Crystallographica , C58, 2002, pp. I31-i32, doi : 10.1107 / S0108270101021928 .
- ↑ a b D. Mootz, H. Rütter: Hydrates of weak and strong bases. VII: On the cesium hydroxide-water system: The crystal structures of CsOH · 2 H 2 O and CsOH · 3 H 2 O. In: Zeitschrift für inorganic und Allgemeine Chemie , 608 (2), 1992, pp 123-130, doi : 10.1002 /zaac.19926080218 .
- ↑ Entry on cesium compounds. In: Römpp Online . Georg Thieme Verlag, accessed on July 14, 2014.





