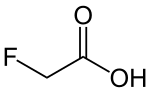
Fluoroacetic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Fluoroacetic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 2 H 3 FO 2 | ||||||||||||||||||
| Brief description |
colorless and odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 78.04 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.37 g cm −3 (36 ° C) |
||||||||||||||||||
| Melting point |
35 ° C |
||||||||||||||||||
| boiling point |
165 ° C |
||||||||||||||||||
| Vapor pressure |
5.3 h Pa (20 ° C) |
||||||||||||||||||
| pK s value |
2.59 (25 ° C) |
||||||||||||||||||
| solubility |
easily in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Fluoroacetic acid is a fluorinated organic compound from the group of carboxylic acids . The highly toxic substance is made to fight rodents . In poison bait it is not usually the free acid that is used, but its salts ( monofluoroacetate ). The colorless, crystalline substance decomposes when heated.
Occurrence
In nature, the sodium salt sodium fluoroacetate is found as a poisonous ingredient in the leaves of a South African shrub “Gifblaar” (“poison leaf”, Dichapetalum cymosum ). Eating the plant can lead to livestock poisoning.
Extraction and presentation
Fluoroacetic acid can be obtained by reacting methyl iodoacetate and silver (I) fluoride or methyl chloroacetate and potassium fluoride followed by ester hydrolysis.
toxicology
Fluoroacetic acid substitutes acetic acid in the citric acid cycle and leads to enzyme blockade at the citrate / cis-aconite level (via the metabolite fluorocitrate ) and is therefore highly toxic. The lethal dose for humans is about 5 mg kg −1 . In rodents such as mice or rats , which have a correspondingly higher metabolism , the dose is significantly lower at 0.1 mg · kg −1 . In contrast, the compound is almost non-toxic to fish and aquatic invertebrates.
Individual evidence
- ↑ a b c d e f g h Entry on fluoroacetic acid in the GESTIS substance database of the IFA , accessed on November 17, 2019(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dissociation Constants of Organic Acids and Bases, pp. 8-42.
- ↑ Entry on fluoroacetic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ R. v. Ostertag, G. Kulenkampff: Animal diseases and herd diseases in Africa. W. de Gruyter, 1941, DNB 362284067 .
- ^ Victor H. Agreda, Joseph R. Zoeller (Eds.): Acetic Acid and Its Derivatives. Marcel Dekker, New York et al. 1992, ISBN 0-8247-8792-7 .
- ↑ Yakkyoku, in Pharmacy. 28/1977, pp. 329-339. (in Japanese).
- ^ JC Ward: Rodent control with 1080, ANTU, and other war-developed toxic agents. In: Am J Public Health Nations Health. 36/1946, pp. 1427-1431.
- ^ Pest Management Regulatory Agency, Re-evaluation of Sodium Monofluoroacetate. June 18, 2004 (PDF; 92 kB).



