Sodium fluoroacetate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium fluoroacetate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 2 FNaO 2 | |||||||||||||||
| Brief description |
colorless odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 100.02 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
200 ° C (decomposition) |
|||||||||||||||
| solubility |
easily soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
DFG / Switzerland: 0.05 mg m −3 (measured as inhalable dust ) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium fluoracetate , also known as sodium monofluoroacetate , is the colorless and highly toxic sodium salt of fluoroacetic acid with the constitutional formula CH 2 FCOONa.
Manufacturing
Sodium fluoracetate is formed, for example, when sodium hydroxide solution , sodium carbonate or sodium hydrogen carbonate react with fluoroacetic acid.
properties
Sodium fluoroacetate is a colorless and odorless powder that is easily soluble in water. The melting point is 200 ° C.
toxicity
Sodium fluoroacetate is extremely toxic to humans, all mammals and many insects, both when ingested orally and when the dusts or mists of aqueous solutions are inhaled, as well as when in direct contact with the skin. After ingesting the poison, the first symptoms of poisoning appear in humans after about 30 minutes to a maximum of three hours . Nausea, vomiting along with abdominal pain, as well as sweating, confusion and restlessness. In addition, there are usually muscle cramps, shortness of breath and cyanosis . Death is usually caused by a ventricular tachycardia .
For humans, the lethal dose was extrapolated to 5 to 10 mg kg −1 body weight.
Subletal doses lead to tissue damage in organs with increased energy requirements, especially in the brain, the gonads , heart and lungs.
Cats can poison themselves from eating poisoned rodents (see Usage ). The lethal dose LD 50 for cats is 0.3 to 0.5 mg kg −1. For various bird species such as house sparrows and starlings , LD 50 values of 2 to 3 mg kg −1 were determined. In guinea pigs the lethal dose 50 is 0.3 mg kg −1 .
For aquatic invertebrates (EC 50 = 350 mg a.i./liter) as well as for fish (LC 50 = 54–970 mg a.i./liter) the compound is almost non-toxic (EC = effective concentration, LC = lethal concentration).
The high toxicity, the easy procurement or the simple production of sodium fluoroacetate make this compound a potential weapon in the hands of terrorists.
Mechanism of action
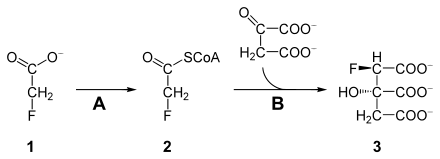
After recording is sodium fluoroacetate ( 1 ) in the body (2 R , 3 S ) -Fluorcitrat ( 3 ) metabolized (see. Figure). This derivative of citrate inhibits the enzyme aconitase , which normally catalyzes the conversion of citrate to cis-aconitic acid . This interrupts the citric acid cycle and citrate accumulates in the body. The body cells are cut off from the energy supply. Cells with high energy requirements are the most affected.
Antidotes
The treatment of poisoning with sodium fluoroacetate is very difficult due to the intervention in the citric acid cycle. As soon as the poisoned person shows symptoms, the cycle is deactivated. There is no known effective antidote . In the clinic, the administration of muscle relaxants, an anticonvulsant and artificial ventilation are helpful as supportive measures. There is little experience with the treatment of such poisoning.
use
Sodium fluoroacetate is approved as a rodenticide for the control of rodents and other mammals in many countries . In New Zealand it is used against possums , in the USA against coyotes . It is also used in Australia , Mexico and Israel . In Canada , it is used against wolves and coyotes in the provinces of Alberta and Saskatchewan .
Some publications show the advantageous use of 18 F-fluoroacetates as tumor markers for imaging in positron emission tomography of prostate carcinomas . Since only picomolar amounts of a tracer are required for such recordings , the toxicity of sodium fluoroacetate is irrelevant in this case. The process is still in the development phase.
Natural occurrence

The salt is found in over 40 plant species in Australia , Brazil and Africa . For example, it occurs in the leaves of the South African plant species "Gifblaar" ( Dichapetalum cymosum ). It is responsible for a wide variety of livestock poisoning. About 30 g are enough to kill a sheep.
It is one of the few natural organofluorine compounds. The compound could also be detected in tea leaves in extremely small amounts .
Individual evidence
- ↑ a b c d e f Entry on sodium fluoroacetate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on Sodium fluoroacetate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 62-74-8 or sodium fluoracetate ), accessed on November 2, 2015.
- ↑ Yakkyoku, in Pharmacy , 28/1977, pp. 329-39. (in Japanese).
- ^ A b Ward JC, Rodent control with 1080, ANTU, and other war-developed toxic agents. , in Am J Public Health Nations Health , 36/1946, pp. 1427-1431, PMC 1624511 (free full text).
- ↑ Fluoridealert.org, Sodium fluoroacetate , accessed December 31, 2007 .
- ↑ Wilfried Kraft: Cat diseases. Schlütersche, 2003, ISBN 978-3-7944-0199-4 , p. 121 ( limited preview in the Google book search).
- ↑ fluoridealert.org, Sodium Fluoroacetate (also known as 1080) , accessed December 31, 2007 .
- ↑ a b Pest Management Regulatory Agency, Re-evaluation of Sodium Monofluoroacetate , June 18, 2004 (PDF; 92 kB).
- ↑ Holstege CP et al., Unusual but potential agents of terrorists. In: Emerg Med Clin North Am. 25/2007, pp. 549-566, PMID 17482032 .
- ^ A b Proudfoot AT et al., Sodium fluoroacetate poisoning. In: Toxicol Rev. 25/2006, pp. 213-219, PMID 17288493 .
- ↑ Sodium fluoroacetate (Compound 1080). US EPA Fact Sheet. August 1990 .
- ↑ Ponde DE et al., 18F-fluoroacetate: a potential acetate analog for prostate tumor imaging - in vivo evaluation of 18F-fluoroacetate versus 11C-acetate. In: J Nucl Med. 48/2007, pp. 420-428, PMID 17332620 .
- ^ GW Gribble: Progress in the Chemistry of Organic Natural Products . Springer Science & Business Media, 2012, ISBN 978-3-7091-6887-5 , p. 108 ( limited preview in Google Book search).
- ↑ T. Vartiainen, P. Kauranen: The determination of traces of fluoroacetic acid by extractive alkylation, pentafluorobenzylation and capillary gas chromatography-mass spectrometry . In: Analytica Chimica Acta . tape 157 , 1984, pp. 91-97 , doi : 10.1016 / S0003-2670 (00) 83609-0 .
literature
- F. Guardian: Modern rodent killers . In: Scoreboard for pest science . tape 23 , no. 4 , April 1950, p. 49-51 , doi : 10.1007 / BF02330830 .
- Goh CS et al., Sodium monofluoroacetate (Compound 1080) poisoning in dogs. , in Aust Vet J. , 83/2005, pp. 474-479. PMID 16119418