Constitutional formula
The constitutional formula is a chemical representation. The constitutional formula can be used to express how the atoms of a molecule are connected to one another by chemical bonds .
| Structural formulas | Other modes of representation | ||||||
|---|---|---|---|---|---|---|---|
| Electron formula | Valence stroke formula | Wedge formula | Skeletal formula | Constitutional formula | Molecular formula | Ratio formula | |
| methane |

|

|
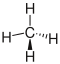
|
does not exist | CH 4 | CH 4 | CH 4 |
| propane |

|

|

|
|
CH 3 -CH 2 -CH 3 | C 3 H 8 | C 3 H 8 |
| acetic acid |

|

|

|

|
CH 3 -COOH | C 2 H 4 O 2 | CH 2 O |
| water |
|
|
|
does not exist | does not exist | H 2 O | H 2 O |
In contrast to the sum formula and the ratio formula , from which only the number and / or numerical ratio of the atoms of different elements can be derived, the constitutional formula also provides topological information from which, for example, the assignment of the represented substance to a compound class can be determined. The constitutional formula thus corresponds to the semi- structural formula (group formula ) in the true sense.
Further information on the spatial composition of a molecule is provided by structural formulas such as bsw. the valence line formula . The terms structural formula, valence formula and constitutional formula are often confused with each other and used congruently.
Edward Frankland was one of the first chemists to use constitutional formulas to represent chemical compounds in a textbook in 1866. Constitutional formulas were one of the first applications of early mathematical graph theory .
Single receipts
- ↑ Brink, Klaus: DIN 32641 - Chemical formulas, in Praxis der Naturwissenschaften-Chemie, 2/49, 2000, page 16f.
- ^ Edward Frankland: Lecture Notes for Chemical Students . Van Voorst, London, 1866, pp. 23–26 ( full text in the Google book search).
- ^ Norman L. Biggs, E. Keith Lloyd, Robin J. Wilson: Graph Theory 1736-1936 . Oxford University Press, 1999, ISBN 0-19-853916-9 .
- ↑ James Joseph Sylvester: Chemistry and Algebra . In: Nature . Volume 17, p. 284.
- ^ Arthur Cayley: Chemical Graphs . In: Philosophical Magazine . Volume 47, 1874, pp. 444-446.