Electron formula
The electron formula (also Lewis formula or Lewis structure , after Gilbert Newton Lewis ) is a chemical structural formula . It is used to represent atoms in molecules , which shows the structure of the molecules. The electron formula is a symbol notation used to describe the structure of a molecule, specifying the individual atoms and valence electrons .
| Structural formulas | Other modes of representation | ||||||
|---|---|---|---|---|---|---|---|
| Electron formula | Valence stroke formula | Wedge formula | Skeletal formula | Constitutional formula | Molecular formula | Ratio formula | |
| methane |

|

|
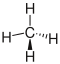
|
does not exist | CH 4 | CH 4 | CH 4 |
| propane |

|

|

|
|
CH 3 -CH 2 -CH 3 | C 3 H 8 | C 3 H 8 |
| acetic acid |

|

|

|

|
CH 3 -COOH | C 2 H 4 O 2 | CH 2 O |
| water |
|
|
|
does not exist | H 2 O | H 2 O | H 2 O |
construction
In the Lewis structure is the element symbol for the atomic core , ie for the atomic nucleus to the inner electron shells . The one to eight bound and unbound valence electrons in the outermost electron shell of the atom are represented by dots. The electrons are distributed as far as possible in four positions around the element symbol. The brief representation in the electron formula fulfills its purpose sufficiently, since only the valence electrons of the atoms are relevant for chemical reactions or chemical bonds .
Atoms can be combined into molecules using the electron formula. If the atomic composition of small molecules is known, the molecular structure of a compound can be predicted.
Differentiation from the valence line formula
In contrast to the valence line formula , the valence electrons of the non-metal atoms are shown individually and not as a pair to form so-called valence lines.
The distinction between the valence formula and the electron formula is not uniform. In some places the term "electron formula" is even used synonymously for "valence line formula". This is also called the "Lewis electron formula".
As a mixed form, there is also the notation that a line replaces two outer electrons (free electron pair , non-binding electron pair) that occupy the same orbital . Points, on the other hand, symbolize individual electrons of an electron pair, which is not possible in the valence notation.
See also
Individual proof
- ↑ Entry on Lewis formula (electron dot or Lewis structure) . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.L03513 Version: 2.1.3.
literature
- Gilbert Newton Lewis: Valence and the structure of atoms and molecules. 1923, ISBN 0486615553 .
- Gilbert Newton Lewis: The Atom and the Molecule. In: J. Am. Chem. Soc. 38. 1916, 762-785, doi : 10.1021 / ja02261a002 .