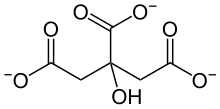
Citrates
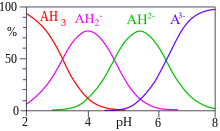
Citrate or citrates are the esters , salts , and the anion of citric acid . In biochemistry, citrates are used when referring to the dissociated ionic form of citric acid that occurs in the aqueous environment of a cell . In aqueous solutions with a neutral pH value, citrate is mainly present as a triple anion. Citrates are metabolizable chelators .
Biological importance
Citrates occur in the metabolism to generate energy via the citric acid cycle . Citrate is metabolized to isocitrate or split into oxaloacetate and acetyl-CoA . It occurs in the blood plasma in a concentration of 0.05 to 0.3 mM . Citrates are also the most common organic acid anions in urine . Citrates are considered to be one of the most important inhibitors of the formation of calcium-containing urinary stones . Decreased levels of citrate in the urine increase the risk of the formation of urinary stones. The citrate excreted in the urine comes from the metabolism (citric acid cycle) on the one hand, and is ingested with food on the other hand, with 65 to 90% of the citrates in humans usually being absorbed in the kidneys .
use
Citrates are used as chelators to supply living things with various metal ions . Furthermore, they are used in biochemistry as buffers ( citrate buffer and phosphate-citrate buffer ), e.g. B. to store blood as citrated blood while avoiding blood clotting . If a chelating effect is undesirable, instead of citrate buffers (or the likewise chelating tartrate , phthalate and phosphate buffers ) z. B. acetate buffer or from the group of Good buffers MES , MOPS and PIPES buffers are used. In the pharmaceutical industry , citrates are approved pharmaceutical excipients for intravenous injection solutions up to a mass fraction of 0.72%.
- Sodium citrate is a component of the Benedict reagent for the detection of reducing sugars and is used for anticoagulation in citrate dialysis .
- Potassium citrate is used in medicine, food technology (E 332) and in the cosmetics and pharmaceutical industries.
- Magnesium citrate is said to increase the magnesium level in the body as a preparation or as a dietary supplement and thus, for example, prevent leg cramps and increase general performance.
- Calcium citrate- containing preparations are used as dietary supplements in the wellness industry. Similar preparations are also given to dogs to strengthen their teeth and bones.
- Ammonium iron (III) citrate is used in medicine to treat iron deficiency anemia and was historically significant in the cyanotype .
- Copper citrate is used in oenology to treat the Böckser wine fault , it also serves as an algicide , as a pigment and is also a component of some food supplements .
- Silver citrate is a component of disinfecting and bactericidal formulations , e.g. B. in foot spray.
- The salts trisodium citrate and trilithium citrate are used in construction chemistry - depending on the amount added - as a retarder or accelerator for the hardening of cementitious compounds .
- Many basic active pharmaceutical ingredients are presented as citrates (for example, sildenafil citrate in Viagra ).
Individual evidence
- ^ A b c Richard P. Lifton: Genetic Diseases of the Kidney. Academic Press, 2009, ISBN 978-0-080-92427-4 , p. 203.
- ↑ Jeremy M. Berg: Stryer Biochemistry. Springer-Verlag, 2015, ISBN 978-3-827-42989-6 , p. 1135.
- ↑ a b Esmail Koushanpour: Renal Physiology. Springer Science & Business Media, 2013, ISBN 978-1-475-71912-3 , p. 228.
- ^ W. Ternes: Biochemistry of the elements. Springer-Verlag, 2012, ISBN 978-3-827-43020-5 , p. 217.
- ↑ Werner A. Eckert: Proteins: Standard Methods in Molecular and Cell Biology. Springer-Verlag, 2013, ISBN 978-3-642-59227-0 , p. 22.
- ↑ Axel M. Gressner: Lexicon of Medical Laboratory Diagnostics . Springer-Verlag, 2013, ISBN 978-3-642-12921-6 , p. 10.
- ↑ Z. Marczenko: Separation, Preconcentration and Spectrophotometry in Inorganic Analysis. Elsevier, 2000, ISBN 978-0-080-54108-2 , p. 44.
- ↑ Safaraz K. Niazi: Handbook of Pharmaceutical Manufacturing Formulations. CRC Press, 2016, ISBN 978-1-420-08131-2 , p. 160.
- ↑ Amitava Majumder, Anne Paschen: Medical working techniques. In: Jörg Braun, Roland Preuss (Ed.): Clinic Guide Intensive Care Medicine. 9th edition. Elsevier, Munich 2016, ISBN 978-3-437-23763-8 , pp. 29–93, here: p. 65.