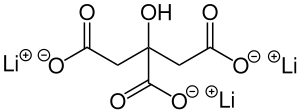
Lithium citrate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Lithium citrate | |||||||||
| other names |
|
|||||||||
| Molecular formula | Li 3 C 6 H 5 O 7 | |||||||||
| Brief description |
white solid (tetrahydrate) |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | ||||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
|
|||||||||
| solubility |
easily soluble in water (209.8 g l −1 at 20 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Lithium citrate is a chemical compound of lithium from the citrate group .
Extraction and presentation
Lithium citrate can be obtained by reacting citric acid with lithium carbonate .
properties
Lithium citrate tetrahydrate is a white solid that is readily soluble in water.
use
Lithium citrate is used in medicine against gout and in neurology.
Individual evidence
- ↑ a b c d e f data sheet Lithium citrate tribasic tetrahydrate, BioUltra, ≥99.5% (NT) from Sigma-Aldrich , accessed on August 2, 2017 ( PDF ).
- ↑ Lithium citrate data sheet (PDF) from Merck , accessed on August 2, 2017.
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 92nd Edition . CRC Press, 2011, ISBN 978-1-4398-5512-6 , pp. 108 ( limited preview in Google Book search).
- ↑ Paul Koenig: Joh. Karl Koenig's dictionary of goods for the trade in drugs and chemicals With Latin, German, English, French, Dutch and Danish names . Springer-Verlag, 2013, ISBN 978-3-663-02608-2 , pp. 324 ( limited preview in Google Book search).
- ↑ Spectrum Academic Publishing House: Lithium Citrate