Aconitase
| Aconitase | ||
|---|---|---|

|
||
| Fig. 1: Tertiary structure of the bovine mAc, Bos taurus , with bound α-methyl isocitrate (calotte, right) next to Fe-S clusters according to PDB 1AMI | ||
| Properties of human protein | ||
| Mass / length primary structure | 889 amino acids | |
| Secondary to quaternary structure | Monomer | |
| Cofactor | [4Fe-4S] | |
| Identifier | ||
| Gene names | ACO1 ; IREB1; IRP1; IREBP; ACO2 | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 4.2.1.3 , lyase | |
| Response type | Rearrangement | |
| Substrate | Citrate | |
| Products | Isocitrate | |
| Occurrence | ||
| Homology family | cyt. Aconitase | |
| Parent taxon | Creature | |
Aconitase (ACO) (more precisely: aconitate hydratase ) are enzymes that catalyze the conversion of citrate or isocitrate into aconitate and vice versa . It is an indispensable enzyme of the citric acid cycle and the glyoxylate cycle . Aconitase is found in all eukaryotes and bacteria in the cytosol . Multicellular animals have an additional copy of the ACO in the mitochondrion . In mammals and bacteria eventually the cytosolic ACO has evolved and the additional role of translation - repressor assumed a central role in iron metabolism plays. Mutations in the iron-sulfur cluster scaffold protein (ISCU) can lead to the rare disease aconitas deficiency, as the ACO function depends on the presence of the cluster.
The catalyzed equilibrium is:
Citrate and aconitate are converted into one another (1), like aconitate and isocitrate (2). The control of the overall reaction from citrate to isocitrate is determined by how the aconite molecule binds to the enzyme, for which there are two possibilities (see picture below).
Aconitase-1 and -2 are the outdated names for the two aconitases in animals (cytosolic, cAc, and mitochondrial, mAc). Bacterial aconitases are called AcnA and AcnB. cAc and AcnA are orthologous to one another . So in total there are three phylogenetic categories.
The presence of the iron-sulfur cluster - cofactor determines the function of the aconitase -1. In the citric acid cycle, it thus catalyzes the reversible rearrangement of the citrate via the intermediate enzyme-bound cis -aconitate to form isocitrate . Aconitase-1 without the iron cluster binds to iron response elements (IRE) in several RNAs and thus regulates the translation of ferritin , the δ-aminolevulinate synthase and the transferrin receptor (Fig. Below).

biosynthesis
The human ACO1 - gene is located on chromosome 9 and extends over 66,220 base pairs and 21 exons . The mRNA is 3,533 bases long and the protein with 889 amino acids is created through translation and post-translational modification .
The ACO2 gene is on chromosome 22 and the final protein contains 753 amino acids.
structure
The protein folds into four domains, three of which are closely linked. The fourth forms a pocket with the other three in which catalysis takes place. The specific conformation of the enzyme with the [4Fe-4S] cluster and several amino acid residues is responsible for the catalytic activity, which allows the reaction to proceed stereospecifically from the achiral citrate exclusively to the (1 S , 2 R ) -isocitrate.
Mitochondrial aconitase contains, bound to the cysteine residues -385, -458 and -461, an iron-sulfur cluster [4Fe-4S], which is crucial for the catalytic activity. In the inactive state, the cluster lacks the fourth iron atom, this is only loosely bound and initially has the coordination number 4: three sulfur atoms and a hydroxide ion (from water) as a binding partner (see Fig. 2). In the catalytic phase it increases to 6, then isocitrate and another water molecule are also bound.
Fig. 1 shows the tertiary structure determined by X-ray crystallography of the mAc with bound α-methyl isocitrate (an ACO inhibitor). In the active center there is the [4Fe-4S] cluster, which, together with side chains of polar amino acids, mainly arginine residues, binds the substrate.
Catalyzed equilibrium
Citrate is converted to cis aconitate and this to L - threo isocitrate (1 S , 2 R isocitrate), and vice versa. The last step is stereoselective . Under standard conditions , 91% citrate, 3% cis -aconitate and 6% isocitrate are in equilibrium. The following explanations on the course of the reaction were obtained exclusively from studies on mitochondrial aconitase, but due to the similarity of the structure they can be transferred to cytosolic ACO.
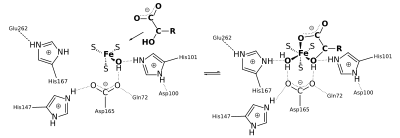
If we start from the isocitrate side, isocitrate is first bound to the fourth iron atom of the cluster by means of two of its hydroxyl groups (see Fig.). The hydroxide previously bound to the iron changes its hydrogen bond from Asp-100 to His-167. Asp-100 now forms a bridge to the hydroxyl of isocitrate. The hydrogen atoms of the hydroxide and the hydroxy group in turn form bridges to the oxygen atoms of Asp-165.
The deprotonated Ser-642 causes extensive electron transfer to His-101. Ser-642 receives a proton and the iron atom receives a second hydroxyl group (see Fig.), Which are reused in the second part of the reaction, the hydration of aconitate. Asp-165 is crucial for the binding of the hydroxyl groups on the iron atom.
literature
- M. Claire Kennedy and Helmut Beinert: IX.4. Aconitase . In: Ivano Bertini, Harry B. Gray, Edward I. Stiefel, Joan Selverstone Valentine (Eds.): Biological Inorganic Chemistry: Structure and Reactivity . University Science Books, Herndon 2006, ISBN 1-891389-43-2 , pp. 209 ff .
Individual evidence
- ^ Orphanet Aconitas shortage
- ↑ aconitase. In: Online Mendelian Inheritance in Man . (English).
- ↑ ENSEMBL entry
- ↑ UniProt Q99798
- ↑ Robbins AH, Stout CD: Structure of activated aconitase: formation of the [4Fe-4S] cluster in the crystal . In: Proc. Natl. Acad. Sci. USA . 86, No. 10, May 1989, pp. 3639-3643. PMID 2726740 . PMC 287193 (free full text).