toluene
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
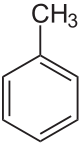
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | toluene | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 7 H 8 | ||||||||||||||||||
| Brief description |
colorless liquid with a characteristic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 92.14 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.87 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−95 ° C |
||||||||||||||||||
| boiling point |
111 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
poor in water (520 mg l −1 at 20 ° C) |
||||||||||||||||||
| Dipole moment | |||||||||||||||||||
| Refractive index |
1.4969 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
DFG / Switzerland: 50 ml m −3 or 190 mg m −3 |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Toluene , common name according to IUPAC also toluene , methylbenzene , phenylmethane , called methylbenzene according to IUPAC nomenclature , is a colorless, characteristic-smelling, volatile liquid that is similar to benzene in many of its properties . Toluene is an aromatic hydrocarbon ; it often replaces the toxic benzene as a solvent . It is also contained in gasoline , among other things .
etymology
As Henri Etienne Sainte-Claire Deville toluene from Tolu won, he had adopted the name Benzoën for this body, recalls in the balms of which he comes to the almost generic character, namely to keep the acid, benzoic acid. Berzelius then suggested the name Toluin in his annual reports . The modification in toluene appears for the first time in a work by Muspratt and Hoffmann , who, however - probably erroneously - refer to the above passage by Berzelius with regard to the name.
history
Toluene was first isolated by Pierre-Joseph Pelletier and Filip Neriusz Walter in 1837. Pelletier found the compound in the gas that is emitted by the bark of the maritime pine (then Pinus maritima ). Pelletier named the substance retinaphta , after the pine resin . In 1841, Henri Etienne Sainte-Claire Deville obtained toluene from the balsam of tolu by dry distillation ; The present name is based on this. By nitrating toluene using nitrating acid (mixture of nitric acid and sulfuric acid ), the chemist Julius Wilbrand produced TNT for the first time in 1863 . Large-scale production of TNT finally started in Germany in 1901; this procedure is still used today.
Occurrence and emissions
Toluene occurs in small quantities in crude oil and in the light oil that is obtained from coal tar distillation . Since petrol contains toluene, it is released in motor vehicles, among other things. It is produced in small quantities during the incomplete combustion of organic substances, such as smoking. There has been a decline in toluene emissions in recent years. For example, the annual mean in Rhineland-Palatinate is 30 µg / m 3 ; depending on the location, however, there may be greater fluctuations in this value. The main emission factor is motor vehicle traffic with around 65%, 33% are due to the use of toluene products and 2% to the manufacture of toluene. Released toluene is broken down in the earth's atmosphere, as with benzene, after several days through reactions with hydroxyl radicals (OH radicals).
Extraction and presentation
A direct extraction from crude oil or by dry distillation of hard coal - based on the crude oil prices in 2006 - is not yet economical.
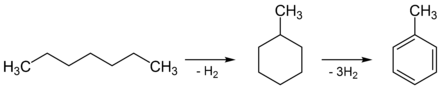
In the industry, there is obtained mainly in the processing of petroleum by the (by cracking generated) n -heptane to methylcyclohexane reformed and is then dehydrogenated to toluene. This process is also called dehydrocyclization .
During the Second World War there were bottlenecks in the production of toluene in Germany due to the lack of petroleum, which is why it was also made from benzene and methanol by means of Friedel-Crafts alkylation . Friedel-Crafts alkylation has limiting factors which considerably reduce the yield and thus its economic importance: alkylbenzenes are more reactive than benzene itself in electrophilic substitution on aromatics; therefore the recently formed alkylbenzene tends to react to form two or more alkylated products. The yield of toluene decreases:
In fact, immense amounts of the starting materials are used, since they are inexpensive, and the toluene formed is continuously isolated. Because the chemical processes all take place in the form of equilibrium reactions and are thereby shifted towards toluene.
Toluene is also obtained in the production of ethene and propene . World production is between five million and ten million tons annually. It is accessible photochemically by isomerization of cycloheptatriene .
properties
Physical Properties
Toluene is the simplest representative of the alkylbenzenes . In the air it burns incompletely with a yellow, strongly sooting flame. The liquid smells characteristic, pungent pleasantly (similar to benzene ) and has an odor threshold of 0.6–263 mg / m 3 . Toluene melts at −95 ° C, boils at 111 ° C and, under normal conditions, is a colorless, clear, water-white liquid that is highly refractive ( refractive index : 1.4969). In water , it is almost insoluble (0.47 g / l); it can be mixed in any ratio with carbon disulphide , ethanol and diethyl ether . Toluene is also readily soluble in chloroform , acetone and most other organic solvents. The compound forms azeotropically boiling mixtures with a number of solvents . The azeotropic compositions and boiling points can be found in the following table. No azeotropes are formed with n- hexane , n- heptane , n- octane , benzene, ethylbenzene , cyclohexanol , chloroform, carbon tetrachloride , acetone, methyl ethyl ketone , diethyl ether, ethyl acetate , dimethylformamide , dimethyl sulfoxide , carbon disulfide and phenol .
| Azeotropes with various solvents | ||||||||||||
| solvent | water | Methanol | Ethanol | 1-propanol | 2-propanol | 1-butanol | iso- butanol | sec -butanol | ||||
| Toluene content | in% | 80 | 31 | 32 | 51 | 31 | 68 | 55 | 45 | |||
| boiling point | in ° C | 85 | 64 | 77 | 93 | 81 | 106 | 101 | 95 | |||
| solvent | Ethanediol | Methyl glycol | Ethyl glycol | 1,4-dioxane | Acetonitrile | acetic acid | Pyridine | Methyl isobutyl ketone | ||||
| Toluene content | in% | 93 | 74 | 89 | 20th | 24 | 72 | 68 | 97 | |||
| boiling point | in ° C | 110 | 106 | 110 | 102 | 81 | 101 | 108 | 111 | |||
The dynamic viscosity is 0.6 mPa · s, so toluene is less fluid than water. The calorific value is 40,940 kJ / kg.
Compilation of the most important thermodynamic properties property Type Value [unit] Remarks Standard enthalpy of formation Δ f H 0 liquid
Δ f H 0 gas12.0 kJ mol −1
50.1 kJ mol −1as a liquid
as a gasEnthalpy of combustion Δ c H 0 gas −3910.9 kJ mol −1 Heat capacity c p 157.09 J mol −1 K −1 (25 ° C)
1.70 J g −1 K −1 (25 ° C)
137.2 J mol −1 K −1 (120 ° C )
1.49 J g −1 K −1 (120 ° C)as a liquid
as a gasCritical temperature T c 591.75 K Critical pressure p c 41.58 bar Critical volume V c 0.316 l mol −1 Critical density ρ c 3.17 mol·l −1 Acentric factor ω c 0.264 Enthalpy of fusion Δ f H 6.61 kJ mol −1 at the melting point Enthalpy of evaporation Δ V H 0
Δ V H38.1 kJ mol −1
33.18 kJ mol −1
at normal pressure boiling point
Chemical properties
Toluene is stable and relatively inert under normal conditions. It reacts similarly to phenol and benzene . Toluene attacks many plastics and is therefore usually stored in glass or metal containers. By oxidation (for example with acidic potassium permanganate solution) toluene can benzyl alcohol and benzaldehyde to benzoic acid is converted. Toluene mainly enters into radical substitution reactions and electrophilic substitution reactions . Nucleophilic substitution reactions are less common.
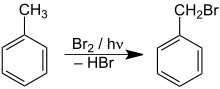
Particularly when exposed to heat or when exposed to light, toluene can enter into radical substitution reactions on the methyl group with suitable reactants (e.g. bromine ) (see SSS rule ):
Since toluene is relatively inert, electrophilic substitution reactions take place relatively slowly on it. In the presence of a suitable catalyst , the reaction rate can be increased considerably (see KKK rule ). There are preferentially formed para - and ortho -substituted products:

Toluene reacts with nitric acid to form 4-nitrotoluene and water, and the isomeric 2-nitrotoluene is also formed. The actual nitrating agent (NO 2 + ) is formed from nitric acid in the presence of sulfuric acid:

TNT (trinitrotoluene) can be obtained through multiple nitration .
Another important reaction is the oxidation of toluene to benzoic acid.
Safety-related parameters
Toluene forms highly flammable vapor-air mixtures. The compound has a flash point of 6 ° C. The explosion range is between 1.1% by volume (42 g / m 3 ) as the lower explosion limit (LEL) and 7.8% by volume (300 g / m 3 ) as the upper explosion limit (UEL). A correlation of the explosion limits with the vapor pressure function results in a lower explosion point of 3 ° C and an upper explosion point of 40 ° C. The limit oxygen concentration is around 9.6% by volume (at 100 ° C). The limit gap width was determined to be 1.06 mm. This results in an assignment to explosion group IIA. The ignition temperature is 535 ° C. The substance therefore falls into temperature class T1. The electrical conductivity of 8 · 10 −14 S · m −1 is very low, so that electrostatic charges can occur when handling.
use
Toluene is used as a solvent and for TNT production. a. used for paints, printing inks , varnishes and adhesives. It is a base chemical for chemical synthesis.
It is also known to be used in permanent markers , which are now mostly produced free of toluene and xylene .
Depending on the measuring range, toluene is also used as a filling liquid in thermometers .
toxicology
Toluene is highly flammable and harmful to health. Toluene causes nerve, kidney and possibly liver damage. Toluene is believed to be harmful to the unborn child. Inhaling toluene vapors can lead to unspecific symptoms such as tiredness, malaise, sensory disturbances, impaired movement coordination and loss of consciousness. Regular contact can lead to toluene addiction, which is accompanied by exhilaration and excitement. Toluene vapors have a narcotic effect and are badly irritating to the eyes and respiratory organs, allergic reactions to toluene are possible. Toluene is believed to be ototoxic in humans. Toluene should be stored in well-ventilated places.
Toluene itself is not mutagenic , but it is often contaminated with benzene. The lower toxicity of toluene compared to benzene can be explained by its different metabolism . In contrast to benzene, toluene is hardly metabolized to benzoic acid by oxidation of the ring, but mainly by oxidation of the side chain. The reason for this is the high selectivity of the monooxygenase system P 450 for the methyl group of toluene. For this reason, hardly any carcinogenic epoxide is produced, as in the case of benzene. The small amounts of epoxide can be degraded by conjugation to glutathione , spontaneous intramolecular rearrangement to the phenol or by enzymatic hydrolysis to the diol.
Toluene and small amounts of o-cresol are excreted in the urine in the form of benzoic acid and hippuric acid .
Risk assessment
Toluene is hazardous to water even in small quantities (WGK 2). It is easily biodegradable. Like benzene, toluene may no longer be used or placed on the market as a substance or component of preparations in over-the-counter adhesives and paint spray cans in the EU.
In 2012, toluene was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. Toluene ingestion was driven by concerns about its classification as a CMR substance, consumer use , high (aggregated) tonnage, other hazard-related concerns and widespread use. The re-evaluation took place from 2012 and was carried out by Finland . A final report was then published.
literature
- LAI impact issues subcommittee: assessment of toluene and xylene immissions. Erich Schmidt Verlag, Berlin 1997, ISBN 3-503-04071-4 .
- Jürgen Angerer: Prevention of occupational health damage caused by benzene, toluene, xylenes and ethylbenzene. Gentner, Stuttgart 1983, ISBN 3-87247-311-5 .
- A. Seeber, M. Blaszkewicz, P. Demes: Toluene in gravure printing. Final report on a research project. HVBG, Sankt Augustin 2002, ISBN 3-88383-623-0 .
- Helmut Greim : Substances that are harmful to health. Toxicological and occupational medical justification of MAK values. Toluene. VCH, Weinheim 1985, ISSN 0930-1984 .
- Methylbenzene, toluene. In: Beilstein's Handbook of Organic Chemistry. Volume 5 (Syst. No. 466), H 280, p. EII 209 .
Web links
- Uses and hazards: André Sepeur: Toluene. In: Umweltlexikon-online.de. February 22, 2012, accessed December 27, 2014 .
- BAUA: Justification for the evaluation of substance properties: Toluene (PDF; 151 kB), May 24, 2002
Individual evidence
- ↑ a b c d e f g h i j k l m n Entry on toluene in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dipole Moments, pp. 9-58.
- ^ Heinz GO Becker, Werner Berger, Günter Domschke: Organikum . 22nd edition. Wiley-VCH, Weinheim 2004, ISBN 3-527-31148-3 , p. 732.
- ↑ Entry on toluene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Schweizerische Unfallversicherungsanstalt (Suva): Limit values - current MAK and BAT values (search for 108-88-3 or toluene ), accessed on November 2, 2015.
- ^ J. Berzelius: Annual report on the progress of chemistry and mineralogy. 22, Lauppsche Buchhandlung, Tübingen 1843, p. 354, online at babel.hathitrust.org, accessed on January 15, 2017.
- ↑ Aug. Wilh. Hofmann: About a safe reaction to benzene. In: Liebigs Ann Chem , 55, 1845, pp. 200-205, doi: 10.1002 / jlac.18450550205 .
- ^ Christian Wiegand: Origin and interpretation of important organic trivial names. I. Benzene series hydrocarbons. In: Angewandte Chemie . 60 (4), 1948, pp. 109-111, doi: 10.1002 / anie.19480600407 .
- ^ H. Limpricht : Textbook of organic chemistry. Volume 2, CA Schwetske & Sohn, 1862, p. 791.
- ↑ Entry on toluene. In: Römpp Online . Georg Thieme Verlag, accessed on March 26, 2017.
- ^ Richard L. Myers: The 100 Most Important Chemical Compounds. Greenwood Press, 2007, ISBN 978-0-313-33758-1 , p. 283.
- ↑ Houben-Weyl : Arene and Arine , 4th edition, Thieme, Stuttgart, 1981, p. 194. Restricted preview in the Google book search
- ↑ a b I. M. Smallwood: Handbook of organic solvent properties. Arnold, London 1996, ISBN 0-340-64578-4 , pp. 40-42.
- ^ A b M. V. Roux, M. Temprado, JS Chickos, Y. Nagano: Critically Evaluated Thermochemical Properties of Polycyclic Aromatic Hydrocarbons. In: J. Phys. Chem. Ref. Data . 37, 2008, pp. 1855-1996.
- ↑ JD Cox, G. Pilcher: Thermochemistry of Organic and Organometallic Compounds. Academic Press, New York 1970, ISBN 0-12-194350-X .
- ^ A b J. PE Grolier, G. Roux-Desgranges, M. Berkane, E. Jimenez, E. Wilhelm: Heat capacities and densities of mixtures of very polar substances 2. Mixtures containing N, N-dimethylformamide. In: J. Chem. Thermodyn. 25, 1993, pp. 41-50.
- ^ A b D. W. Scott: Toluene: thermodynamic properties, molecular vibrations, and internal rotation. In: J. Phys. Chem. 66, 1962, pp. 911-914.
- ↑ a b c d C Tsonopoulos; Ambrose, D .: Vapor-Liquid Critical Properties of Elements and Compounds. 3. Aromatic hydrocarbons. In: J. Chem. Eng. Data . 40, 1995, pp. 547-558. doi: 10.1021 / je00019a002
- ^ Carl L. Yaws, Prasad K. Narasimhan: Thermophysical Properties of Chemicals and Hydrocarbons - Chapter 1: Critical Properties and Acentric Factor, Organic Compounds. 1st edition. Elsevier 2008, ISBN 978-0-8155-1596-8 , p. 31. doi: 10.1016 / B978-081551596-8.50006-7
- ↑ ES Domalski, ED Hearing: Heat Capacities and Entropies of Organic Compounds in the Condensed phase. Volume III. In: J. Phys. Chem. Ref. Data . 25, 1, 1996, doi: 10.1063 / 1.555985 .
- ^ A b V. Majer, V. Svoboda: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation. Blackwell Scientific Publications, Oxford 1985, p. 300.
- ↑ a b c d e E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ Technical rule for hazardous substances TRGS 727, BG RCI leaflet T033 Avoidance of ignition hazards due to electrostatic charges , status August 2016, Jedermann-Verlag Heidelberg, ISBN 978-3-86825-103-6 .
- ↑ Toluene from enius , accessed on February 25, 2018.
- ↑ P. Hoet, D. Lison: Ototoxicity of toluene and styrene: state of current knowledge. In: Crit Rev Toxicol. Volume 38, Issue 2, 2008, pp. 127-170. doi: 10.1080 / 10408440701845443 . PMID 18259983 .
- ↑ Regulation (EC) No. 1907/2006 of December 18, 2006. Oj. L 136, May 29, 2007 (PDF) , Annex XVII.
- ^ European Chemicals Agency (ECHA): Substance Evaluation Report and Conclusion Document .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Toluene , accessed on March 26, 2019.