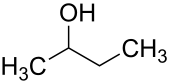
2-butanol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 2-butanol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 4 H 10 O | |||||||||||||||||||||
| Brief description |
colorless liquid with an alcohol-like odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 74.12 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
0.81 g cm −3 |
|||||||||||||||||||||
| Melting point |
−115 ° C |
|||||||||||||||||||||
| boiling point |
99 ° C |
|||||||||||||||||||||
| Vapor pressure |
|
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| Refractive index |
1.3978 (20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
|
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
2-butanol (also sec -butanol or butan-2-ol according to IUPAC ) is a chemical compound and, as a secondary , saturated alcohol, is one of the alkanols .
Isomers
2-Butanol is the only butanol with a center of chirality , so that there are two optically active enantiomers . Whenever “2-butanol” is mentioned in this article or in the scientific literature without additional information on the configuration , the racemate [( RS ) -2-butanol or (±) -2-butanol] is always meant. This racemate previously had the CAS number 15892-23-6, which was deleted and should no longer be used.
| Isomers of 2-butanol | ||
| Surname | ( S ) -2-butanol | ( R ) -2-butanol |
| other names | (+) - 2-butanol | (-) - 2-butanol |
| Structural formula |  |

|
| CAS number | 4221-99-2 | 14898-79-4 |
| 78-92-2 (racemate) | ||
| EC number | 224-168-1 | 238-967-8 |
| 201-158-5 (racemate) | ||
| ECHA info card | 100,021,972 | 100.035.410 |
| 100.001.053 (racemate) | ||
| PubChem | 444683 | 84682 |
| 6568 (racemate) | ||
| DrugBank | - | - |
| DB02606 (racemate) | ||
| Wikidata | Q27104553 | Q70731894 |
| Q209332 (racemate) | ||
Extraction and presentation
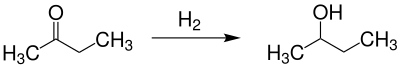
Can be the racemic form of 2-butanol - by - so the mixture of the two enantiomers reduction of 2-butanone obtained:
Another possibility for synthesis is the hydration of but-2-ene .
properties
2-Butanol is a characteristically sweetish and penetrating smelling colorless liquid. Compared to 1-butanol, 2-butanol dissolves better in water, namely at 290 g / l at 25 ° C. It can be mixed with many organic solvents.
Safety-related parameters
2-Butanol is considered a highly flammable liquid. Flammable vapor-air mixtures can form above the flash point. The compound has a flash point at 23 ° C. The explosion range is between 1.65% by volume (51 g / m 3 ) as the lower explosion limit (LEL) and 11% by volume (340 g / m 3 ) as the upper explosion limit (UEL). The lower explosion point is 19 ° C. The ignition temperature is 390 ° C. The substance therefore falls into temperature class T2.
use
2-Butanol is a component in brake fluids, paint strippers and is used as a xanthate for the production of flotation agents . It is also part of mixed solvents. It is also used for the synthesis of esters. This in turn is used in flavors, perfumes and as a solvent for nitrocellulose .
safety instructions
2-butanol is flammable and volatile. When the substance is heated above its flash point, which is 23 ° C (closed cup), the vapors can form an explosive mixture with air. They are heavier than air and can cause drowsiness and drowsiness. Inhaling or swallowing the substance can be harmful to health. 2-Butanol irritates the eyes and the respiratory system. 2-Butanol must not get into the hands of children.
Individual evidence
- ↑ Entry on SEC-BUTYL ALCOHOL in the CosIng database of the EU Commission, accessed on March 11, 2020.
- ↑ a b c d e f g h i j k l m n o p q r s Entry on 2-butanol in the GESTIS substance database of the IFA , accessed on December 27, 2019(JavaScript required) .
- ↑ Entry on butanols. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2014.
- ^ A b Donald B. Alger: The water solubility of 2-butanol: A widespread error. In: Journal of Chemical Education. 68 (11), 1991, p. 939; doi: 10.1021 / ed068p939.1 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-74.
- ↑ Entry on butan-2-ol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 78-92-2 or sec-butanol ), accessed on October 13, 2019.
- ↑ a b c E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.