KKK rule
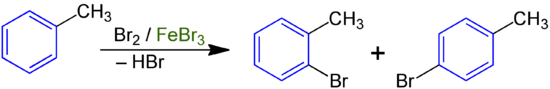
In chemistry, the KKK rule is a rule derived from observations for the regioselectivity in substitution reactions such as chlorination and bromination of alkyl-substituted aromatics such as toluene , ethylbenzene or xylene . It states that, in the case of alkyl-substituted aromatic compounds, a reaction on the aromatic nucleus usually takes place under mild conditions using a catalyst. The three Cs stand for K älte, K atalysator and K s. In English , the rule may as CCC rule be translated ( C old, C atalyst and C ore substitution).
This rule can be derived from the fact that electrophilic aromatic substitutions take place in the presence of catalysts such as aluminum trichloride or iron tribromide , which increase the electrophilicity of the attacking electrophile. The supply of heat would favor a radical reaction process in which aliphatic side chains are preferred reactants.
The counterpart to the KKK rule is therefore the SSS rule , which describes the conditions for radical substitution (boiling heat, sun / radiation) at which aliphatic side chains are attacked but not the aromatic core.
literature
- Reinhard Brückner: reaction mechanisms . 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 218.
Individual evidence
- ↑ dict.cc | CCC rule | Dictionary English German. Retrieved February 22, 2017 .