Methylcyclohexane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
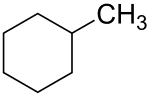
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Methylcyclohexane | |||||||||||||||
| Molecular formula | C 7 H 14 | |||||||||||||||
| Brief description |
colorless liquid with a sweet aromatic smell |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 98.19 g · mol -1 | |||||||||||||||
| density |
0.77 g cm −3 |
|||||||||||||||
| Melting point |
−127 ° C |
|||||||||||||||
| boiling point |
101 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4231 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Methylcyclohexane (hexahydrotoluene, cyclohexylmethane) is an organic- chemical compound from the group of aliphatic hydrocarbons . It belongs to the group of cycloalkanes and is used as a solvent .
Presentation and extraction
Methylcyclohexane occurs in petroleum . A targeted synthesis takes place through the hydrogenation of toluene . Another production variant is the dehydrocyclization of n- heptane . Methylcyclohexane produced in this way is usually further dehydrogenated to toluene.
properties
Physical Properties
Methylcyclohexane is a colorless liquid that smells like petrol and boils at 101 ° C under normal pressure . The heat of vaporization at the boiling point is 31.823 kJ mol −1 . According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in Torr, T in ° C) with A = 7.00206, B = 1375.1330 and C = 232.819 in the temperature range from −3.71 to 127.14 ° C. The odor threshold is between 2000 and 2570 mg · m −3 . It is insoluble in water; it floats on water. In contrast, it dissolves well in alcohol and ether . The dynamic viscosity is 0.67 mPa s, so the liquid is thinner than water, but a little thicker than, for example, toluene .
Safety-related parameters
Methylcyclohexane forms highly flammable vapor-air mixtures. The compound has a flash point of −4 ° C. The explosion range is between 1.1 vol.% (45 g / m 3 ) as the lower explosion limit (LEL) and 6.7 vol.% As the upper explosion limit (UEL). With a minimum ignition energy of 0.27 mJ, vapor-air mixtures are extremely ignitable. The ignition temperature is 260 ° C. The substance therefore falls into temperature class T3.
use
Methylcyclohexane is used as a good solvent for rubber , chlorinated rubber , synthetic rubber , polystyrene , Celluloseester , rosin , Kopalester , Elemi and Pontianak used. It can also serve as a bath liquid for cryostats . Research is also discussing its use as a storage material for hydrogen . At high temperatures it can be reversibly dehydrated to toluene. The hydrogen released in the process could then be used technically.
Safety instructions / risk assessment
When inhaled and swallowed, it leads to dizziness and drowsiness, it reddens the eyes and degreases the skin.
In 2012, methylcyclohexane was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of methylcyclohexane were concerns about consumer use , high (aggregated) tonnage and widespread use as well as the dangers arising from a possible assignment to the group of PBT / vPvB substances. The re-evaluation took place from 2013 and was carried out by Finland . A final report was then published.
Individual evidence
- ↑ a b c d e f g h i j k l m n o p Entry on methylcyclohexane in the GESTIS substance database of the IFA , accessed on April 20, 2018(JavaScript required) .
- ↑ a b c d e f g h Entry on methylcylohexane. In: Römpp Online . Georg Thieme Verlag, accessed on April 20, 2018.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-346.
- ↑ Entry on methylcyclohexane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 108-87-2 or methylcyclohexane ), accessed on November 2, 2015.
- ^ A b Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons , 1st Edition Elsevier 2008, ISBN 978-0815515968 , p. 340.
- ↑ Carl L. Yaws: The Yaws Handbook of Vapor Pressure - Antoine Coefficients , 2nd Edition Elsevier 2015, ISBN 978-0-12-802999-2 , p. 50, doi : 10.1016 / B978-0-12-802999-2.00004- 0 .
- ^ GWH Scherer, E. Newson, "Analysis of the seasonal energy storage of hydrogen in liquid organic hydrides", International Journal of Hydrogen Energy , 1998, 23, 1, 19-25, doi: 10.1016 / S0360-3199 (97) 00018 -9 .
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Methylcyclohexane , accessed on March 26, 2019.




