Liquid organic hydrogen carriers
As liquid organic hydrogen carrier (English: liquid organic carriers hydrogen , LOHC ) refer to organic compounds, the hydrogen absorb by chemical reaction and release it again. LOHCs can therefore be used as storage media for hydrogen .
In principle, any unsaturated compound ( organic molecules with C -C double or triple bonds in) hydrogenation hydrogen record .
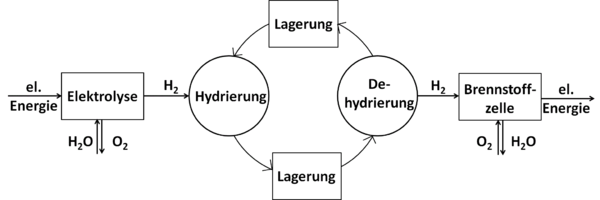
Principle of LOHC-based hydrogen storage
To absorb hydrogen, the dehydrated form of LOHC (an unsaturated, mostly aromatic compound) reacts with the hydrogen in a hydrogenation reaction . The hydrogenation is an exothermic reaction and is carried out at elevated pressures (approx. 30-50 bar) and temperatures of approx. 150-200 ° C in the presence of a catalyst . The corresponding saturated compound is thereby formed, which can be stored or transported under ambient conditions. If the hydrogen is needed again, the now hydrogenated, hydrogen-rich form of the LOHC is dehydrogenated , with the hydrogen being released again from the LOHC. This reaction is endothermic and takes place at elevated temperatures (250-320 ° C) again in the presence of a catalyst. Before the hydrogen can be used, it may have to be cleaned of LOHC steam. To increase efficiency, the heat contained in the hot material flow exiting the release unit should be transferred to the cold material flow consisting of hydrogen-rich LOHC entering the release unit in order to keep the energy requirement for preheating it before the reaction low.
In particular, the heat released by the hydrogenation reaction when the hydrogen is absorbed can in principle be used for heating purposes or as process heat.
Requirements for LOHC materials
The most important requirements for a LOHC are:
- Liquid aggregate state in the entire relevant temperature range
- Temperature and cycle stability
- Reversibility of hydrogen uptake
In addition, the environmental compatibility must be checked depending on the area of application . In closed systems with appropriate safety measures, for example, toxicity plays a subordinate role.
To ensure easy handling (pumpability, etc.), the liquid state is important. It should be noted that an LOHC exists in different forms in the course of the process: the dehydrated (hydrogen-poor) form, the hydrogenated (hydrogen-rich) form and possibly also various intermediate stages. The melting temperature should be well below room temperature for all forms. This is a problem with N- ethylcarbazole, for example , which has a melting point of approx. 70 ° C. in the dehydrated form. In addition, it must be ensured that a transition to the vapor phase is prevented as far as possible. A boiling point below the temperature during the dehydrogenation reaction , the highest temperature in the process, is not sufficient because the presence of the hydrogen reduces the partial pressure of the LOHC in the gas phase and therefore significant evaporation is possible even below the boiling temperature. LOHC materials should therefore always be substances with the lowest possible vapor pressure .
Since a LOHC recycles, i.e. H. is repeatedly charged and discharged with hydrogen, decomposition should occur as little as possible . In addition to the high temperatures in the process, the presence of catalytically active material during the hydrogenation and dehydrogenation must also be taken into account.
The reversibility of the hydrogen uptake under technically meaningful conditions is decisive for the applicability as LOHC. In principle, every unsaturated compound is able to take up hydrogen through hydrogenation . However, since the release is thermodynamically unfavorable, only aromatic compounds are suitable as LOHCs.
Determination of the degree of hydrogenation
The hydrogenation or dehydrogenation reaction does not necessarily lead to complete conversion of the dehydrogenated or hydrogenated LOHC. A partially hydrogenated mixture can therefore arise which is composed of fully hydrogenated, partially hydrogenated and fully dehydrated molecules. Knowledge of the respective degree of hydrogenation is important for practical use. This can be understood as a kind of "state of charge" or energy content of the LOHC. Therefore, ways of determining the degree of hydrogenation are needed. Since a complex laboratory analysis (for example by means of gas chromatography or NMR ) is not an option in technical practice, correlations with other, more easily measured variables are used. In particular, the refractive index and the density are suitable for this.
Examples of LOHC materials
Toluene / methylcyclohexane
As early as the 1980s there were attempts with toluene , which is converted to methylcyclohexane by hydrogenation . The basic idea of this variant came from the USA in 1975 and was further developed in 1979 at the Paul Scherrer Institute in Switzerland together with the ETH Zurich . Even then, the prototype of a truck was built that was powered by hydrogen from the dehydrogenation of methylcyclohexane. The entire circuit is as M ethylcyclohexan- T oluol- H 2 system (MTH).
N -ethyl carbazole
Current research is currently investigating, among other things, N- ethylcarbazole , which was proposed and patented as a hydrogen storage device by the US company Air Products in the mid-2000s . Due to the presence of the nitrogen atom, dodecahydro- N -ethylcarbazole can be dehydrogenated at significantly lower temperatures than, for example, methylcyclohexane. This enables relatively efficient energy storage. Up to 5.8% by weight of hydrogen can be stored in hydrogenated N- ethylcarbazole, which corresponds to a formal energy content of 1.9 kWh / kg. The dehydration takes place at temperatures of approx. 200 to 230 ° C. The relatively high melting point of the dehydrated form of approx. 70 ° C is a challenge.
Dibenzyltoluene
To circumvent the high melting temperature of N -ethylcarbazole and the high vapor pressure of toluene, dibenzyltoluene can be used. This substance is currently already used as a heat transfer oil . Temperatures of approx. 300 ° C are necessary for dehydration. However, dibenzyltoluene is superior to other carrier substances in many physico-chemical properties.
Other potential LOHCs
Benzyltoluene
Benzyltoluene is chemically closely related to dibenzyltoluene and the chemical characteristics are therefore very similar. The use of benzyltoluene is also being examined. However, the significantly higher vapor pressure in comparison to dibenzyltoluene is a disadvantage.
Naphthalene
The naphthalene / decalin system is also discussed in the specialist literature as a carrier material. The disadvantage here, however, is that, just as with N- ethylcarbazole, the low-hydrogen form is a solid at room temperature.
Azaborine
In order to further reduce the temperature required for the release of hydrogen, a boron atom can be built into the ring structure in addition to a nitrogen atom, as is the case with N -ethylcarbazole . Such azaborines can in principle be dehydrated at very low temperatures. However, many questions regarding stability and reversibility are still unanswered.
implementation
On January 29, 2016, the world's first commercial LOHC facility for storing hydrogen in dibenzyltoluene was inaugurated. It was developed and created by Hydrogenious Technologies GmbH . With the help of solar power from a 98 kW p photovoltaic system, hydrogen is generated by means of PEM electrolysis . This is stored in dibenzyltoluene. Hydrogenious Technologies GmbH won the German Business Innovation Prize for the storage process in 2016 .
Hydrogenius LOHC Technologies is a spin-off of the Friedrich-Alexander-Universität Erlangen with headquarters in Erlangen-Bruck. In November 2017, the company delivered the first LOHC systems for commercial applications to the USA and has since become the world market leader in LOHC technology. This means that commercial hydrogen filling stations with over 1,000 kg of stored hydrogen can be implemented on site in densely populated areas or at locations with limited space requirements. The carrier material used, dibenzyltoluene, is flame retardant, but is also classified as hazardous to water and health.
The flexible, simple and inexpensive transport of hydrogen generated with renewable energies (also known as green hydrogen ) using LOHC technology is of crucial importance for the success of the energy transition, because the best locations for generation are usually not there. where the energy, fuel or raw material are needed. With the LOHC technology from Erlangen, it is also possible to set up international and intercontinental supply chains.
Research on the subject of LOHC is currently taking place at the Friedrich-Alexander-Universität Erlangen-Nürnberg , the Helmholtz Institute Erlangen-Nürnberg for Renewable Energies and the Energie Campus Nürnberg .
Individual evidence
- ↑ D. Teichmann, K. Stark, K. Müller, G. Zöttl, P. Wasserscheid , W. Arlt : Energy storage in residential and commercial buildings via Liquid Organic Hydrogen Carriers (LOHC) . Energy & Environmental Science, 2012, 5, 5, 9044-9054, doi: 10.1039 / C2EE22070A .
- ↑ K. Müller, R. Aslam, A. Fischer, K. Stark, P. Wasserscheid, W. Arlt, "Experimental assessment of the degree of hydrogen loading for the dibenzyl toluene based LOHC system", International Journal of Hydrogen Energy, 2016 , 41, 47, 22097-22103, doi: 10.1016 / j.ijhydene.2016.09.196 .
- ^ M. Taube, P. Taube, "A liquid organic carrier of hydrogen as a fuel for automobiles", In: Hydrogen energy progress; Proceedings of the Third World Hydrogen Energy Conference, Tokyo, Japan, June 23-26, 1980. Volume 2. (A81-42851 20-44) Oxford and New York, Pergamon Press, 1981, pp. 1077-1085.
- ^ M. Taube, D. Rippin, DL Cresswell, W. Knecht, N. Gruenenfelder, "A system of hydrogen-powered vehicles with liquid organic hydrides", International Journal of Hydrogen Energy, 1983, 8, 3, 213-225, doi: 10.1016 / 0360-3199 (83) 90067-8 .
- ^ M. Taube, D. Rippin, W. Knecht, D. Hakimifard, B. Milisavljevic, N. Gruenenfelder, "A prototype truck powered by hydrogen from organic liquid hydrides", International Journal of Hydrogen Energy, 1985, 10, 9, 595-599, doi: 10.1016 / 0360-3199 (85) 90035-7 .
- ↑ Overview of energy storage as an element of a secure energy supply. In: Chemical Engineer Technology. 87, 2015, p. 17, doi : 10.1002 / cite.201400183 , there p. 49. - Joint GCC-JAPAN Environment Symposia in 2013 .
- ^ GP Pez, AR Scott, AC Cooper, H. Cheng, FC Wilhelm, AH Abdourazak, "Hydrogen Storage by Reversible Hydrogenation of pi-conjugated substrates" , Patent US 7351395, filed November 4, 2005, granted April 1, 2008.
- ↑ B. Müller, K. Müller, D. Teichmann, W. Arlt, "Energy storage using methane and energy-carrying substances - a thermodynamic comparison", Chemie Ingenieur Technik, 2011, 83, no. 11, 1-13, doi: 10.1002 / cite.201100113 .
- ↑ N. Brückner, K. Obesser, A. Bösmann, D. Teichmann, W. Arlt, J. Dungs, P. Wasserscheid, Evaluation of Industrially Applied Heat-Transfer Fluids as Liquid Organic Hydrogen Carrier Systems , In: ChemSusChem , 2014 , 7, 229-235, doi: 10.1002 / cssc.201300426 PDF .
- ↑ C. Krieger, K. Müller, W. Arlt: Energetic analysis of LOHC systems as thermochemical heat storage. In: Chemical Engineer Technology. 86, 2014, p. 1441, doi : 10.1002 / cite.201450058 .
- ↑ S. Hodoshima, S. Takaiwa, A. Shono, K. Satoh, Y. Saito, "Hydrogen storage by decalin / naphthalene pair and hydrogen supply to fuel cells by use of superheated liquid-film-type catalysis", Applied Catalysis A. : General, 2005, 283, 1-2, 235-242, doi: 10.1016 / j.apcata.2005.01.010 .
- ↑ K. Müller, K. Stark, B. Müller, W. Arlt, "Amine borane based hydrogen carriers: An evaluation", Energy & Fuels , 2012, 26, 6, 3691-3696, doi: 10.1021 / ef300516m .
- ↑ From research to product - product presentation by Hydrogenious Technologies GmbH, February 1, 2016
- ↑ https://www.hydrogenious.net/index.php/de/hydrogen-2/
- ↑ hydrogen storage. Energie Campus Nürnberg, accessed on April 25, 2019 .
- ↑ From the laboratory to the rails - researchers at HI-ERN are planning hydrogen trains with LOHC technology. In: press release. Forschungszentrum Jülich, April 19, 2018, accessed on April 25, 2019 .