Cycloalkanes
The cycloalkanes ( cyclanes , older designation: naphthenes, cycloparaffins ) are a group of substances of ring-shaped, saturated hydrocarbons . The rings can have side chains . In the systematics of organic chemistry , they are counted among the alicyclic compounds . The cycloalkanes without side chains form a homologous series with the general empirical formula C n H 2n , where n ≥ 3. Thus the smallest occurring cycloalkane is cyclopropane .
Historical information
Naturally occurring cycloalkanes ( cyclopentane , cyclohexane , cycloheptane ) were the first from the chemist Vladimir Markovnikov in the naphtha fraction, and naphtha called the Caucasian oil found. The name naphthene , which is occasionally used for all cycloalkanes, comes from this. Usually this imprecise term is only applied to the derivatives of cyclopentane and cyclopentane. It is still used in the petroleum industry today.
Classification
One criterion for classifying cycloalkanes is the size of the carbon ring. Cycloalkanes whose cyclic carbon chain contains three or four carbon atoms are called small, from five to seven as normal, from eight to eleven as medium and more than eleven as larger.
In addition to simple rings of the monocycles, there are cycloalkanes, which consist of several rings connected to one another, they are called polycyclic alkanes. There are again different ways of connecting the rings: A distinction is made between connected (condensed) and bridged polycycles and spiro compounds .
nomenclature
The names of the cycloalkanes are formed from those of the corresponding open-chain alkanes with the same number of carbon atoms with the prefix Cyclo .
Polycyclic alkanes are also named after the alkanes with the same number of carbons. The number of rings is indicated by the prefix bicyclo , tricyclo , etc. The number of carbon atoms between carbon atoms that connect rings (so-called " bridgehead atoms ") is placed in front of the name in descending order in square brackets. Spiro compounds are named in the same way, but instead of the prefix bicyclo , tricyclo , etc., spiro is used. If the molecule has substituents, their location is marked with a number at the beginning, followed by the name of the substituent and finally the name of the cycloalkane ring , separated by a - .
structure
In the structural formula drawings of the cycloalkanes, the rings are shortened by polygons . Cycloalkanes can be thought of as a form of alkane in which the two ends of the carbon chain are linked together. They are not isomers of the alkanes. Like alkanes, they are saturated compounds.
Isomerism
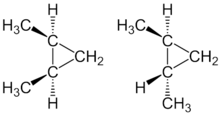
Since the rotation of a substituent around a ring carbon atom is impossible, a special form of isomerism occurs, the cis - trans isomerism . In the case of cis - trans isomers, the spatial arrangement of substituents is different. The substituents can be on the same side ( cis ) or on different sides ( trans ) of the ring bond .
Conformations
In order to avoid molecular tension, the cycloalkanes are not, as often shown, planar. Rather, they are in conformations in which the interior angle of the tetrahedral shape (109.45 °) is retained as far as possible. With cyclopropane , cyclobutane and cyclopentane this angle is not quite reached, so-called Baeyer stress occurs ; because of this tension, these molecules are more reactive. In the cyclohexane molecule, however, this tension no longer has any influence.
properties
The ring-shaped structure of the cycloalkanes affects their reactivity and the melting and boiling points. The melting and boiling points of a monocyclic alkane are always higher than those of the corresponding n -alkane, since the London interaction is stronger with the cycloalkane than with the more flexible n -alkane because of its more rigid structure . The boiling temperature is 3 to 7% higher, the density increases by approx. 15 to 19% and the molar volume decreases to 83% at 20 ° C.
Cyclopropane, cyclobutane and cyclopentane are more reactive than the higher cycloalkanes because the Baeyer stress occurs in them .
Monocyclic unsubstituted alkanes with three or four carbon atoms are gaseous under normal conditions, from five carbon atoms they are liquid.
Cycloalkanes are poorly soluble or insoluble in water. Cycloalkanes are easily flammable, but relatively inert. They essentially undergo the same reactions as the alkanes .
Occurrence
In addition to the six-membered ring of cyclohexane , which occurs in many steroids and terpenes, there are many other derivatives of small rings and macrocycles in nature . The steroids are based on the cycloalkane Gonan .
Many cycloalkanes such as cyclohexane, methylcyclohexane and cyclopentane occur in petroleum. Small amounts of the crystalline solids of the diamondoids , the simplest representative of which is adamantane , were also found there.
Cycloalkanes with a carbon content of 14 to 18 occur, for example, in fragrances such as musk , which is obtained from a gland of the musk deer (Moschidae).
use
Cyclohexane and cyclododecane are starting materials for the synthesis of caprolactam and laurolactam, respectively . Just like the dicarboxylic acids derived from them , these are required for the production of polyamides .
Cyclohexane, decalin , cyclopentane and methylcyclohexane are used as solvents.
Individual evidence
- ↑ Entry on cycloalkanes . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C01497 Version: 2.1.5.
literature
- Josef Houben, Theodor Weyl: Methods of Organic Chemistry, Ln; Methods of organic chemistry, Ln, Bd.5 / 1a, Alkane, Cycloalkane . Thieme, Stuttgart 1994, ISBN 3-13-202204-7