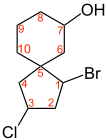Spiro connection
| Spiro compounds |
| Spiro [4.5] decane |
| 1,3,5-Triazaspiro [5.5] -undeca-1,3-diene, example of a heterocyclic spirane. |
| Dispiro [4.2.5.2] pentadecane, example of a dispirane. |
Spiro compounds (Spirane from Latin spira = winding, pretzel) are a class of polycyclic organic compounds whose rings are only connected to one atom. The rings of the spiro compounds can be identical or different. The atom on which the two rings are linked is called the spiro atom . It can be a carbon atom, but also a hetero atom . The smallest spiro compound is spiropentane .
If there are several spiroatoms in one molecule, one speaks of dispirans, trispirans, etc.
Nomenclature of Spiro Compounds
All spiro compounds are preceded by the syllable Spiro , followed by square brackets - [] - the number of ring atoms of the smaller and larger ring (without counting the spiro atom) and the name according to the nomenclature added at the end. The numbering begins with an atom of the smaller ring that is adjacent to the spiro atom and continues over the ring to the spiro atom. Examples:
Chirality
Some spiro compounds show axial chirality (without a chiral center ). It already occurs when the two "brackets" on the spiroatom are the same, but have different beginnings and ends. Although the substitution pattern on carbon is C (AABB), compound C (A – B) 2 is chiral. When determining the configuration according to CIP rules, the question of the priority of A over B or vice versa is irrelevant: You can give any remainder (e.g. A) the higher priority, as well as one of the two brackets (e.g. 1 instead of 2) a slightly higher priority and then determine the configuration ( aR or aS , a = axial) using the priority sequence A1> A2> B1> B2 ; the result is independent of the awards.
In cases with distinguishable brackets (such as 1-bromo-3-chloro-spiro [4.5] decan-7-ol and 1-bromo-3-chloro-spiro [3.6] decan-7-ol above) the order of priority of the four different residues and from this the configuration is determined in the classic manner.
If the spiroatom is not a center of chirality, but the compound is still chiral, then there is a chiral axis that goes through the central spiroatom.
Web links
Individual evidence
- ^ A b Wolfgang Holland: The nomenclature in organic chemistry , VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1969, page 81.
- ^ Philipp Fresenius, Klaus Görlitzer: Organic-chemical nomenclature, Wissenschaftliche Verlagsgesellschaft, Stuttgart, 1991, ISBN 3-8047-1167-7 , pp. 47-49.
- ^ Adalbert Wollrab: Organic chemistry. Springer-Verlag, 2014, ISBN 978-3-642-45144-7 , p. 324.
- ↑ K.-H. Hellwich: stereochemistry. Springer-Verlag, 2007, ISBN 978-3-540-71708-9 . P. 22.