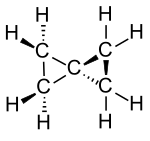
Spiropentane
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Spiropentane | ||||||||||||
| other names |
Spiro [2.2] pentane |
||||||||||||
| Molecular formula | C 5 H 8 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 68.12 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| density |
D 4 20 = 0.7266 g cm −3 |
||||||||||||
| Melting point |
−107.0 ° C |
||||||||||||
| boiling point |
39.5-40.5 ° C at 746 Torr, |
||||||||||||
| Refractive index |
1.4120 (20 ° C) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||
The alicyclic hydrocarbon spiropentane is the simplest representative of the spiro compounds . It contains five carbon atoms, which form two cyclopropane rings that are linked by a common carbon atom. According to the nomenclature rules for spiro compounds, the systematic name Spiro [2.2] is pentane . However, there cannot be any constitutionally isomeric spiropentanes, so the name is clear without brackets and numbers.
Manufacturing
After Gustavson (1887) had obtained cyclopropane from 1,3-dibromopropane by reaction with zinc dust in water-containing ethanol , he applied this method to 2,2-bis (bromomethyl) -1,3-dibromopropane (see equation). This tetrabromide was easy to prepare from pentaerythritol . He received a hydrocarbon with the empirical formula C 5 H 8 , which he initially considered vinyl trimethylene. Fecht expressed the assumption that it must be spiropentane, a constitutional isomer of vinylcyclopropane.
A proof of the structure of the hydrocarbon could be seen in the fact that it could also be obtained from a cyclopropane compound - 1,1-bis (bromomethyl) -cyclopropane - (see equation).
Gustavson's formally simple synthesis was repeated several times. It turned out that the spiropentane produced in this way was not pure, but contained more or less large proportions of other hydrocarbons. Decades later, the manufacturing process was improved; the spiro-hydrocarbon was separated from the by-products (2-methyl-1-butene, 1,1-dimethylcyclopropane, methylenecyclobutane) by rectification and the purity was checked by gas chromatography .
properties
Physical Properties
A structure determination by means of electron diffraction revealed two different C – C bond lengths; the bonds to the quaternary carbon atom (“Spiro-C atom”) are shorter (146.9 pm) than the bonds between the methylene groups (CH 2 –CH 2 , 151.9 pm). The C – C – C angle at the spiro carbon atom is 62.2 °, which is greater than that of cyclopropane .
Chemical properties
When spiropentane labeled with deuterium atoms was heated - as with cyclopropane - a topomerization was observed, also called stereomutation : cis -1,2-dideuteriospiropentane forms trans -1,2-dideuteriospiropentane in an equilibrium reaction.
Gustavson (1896) reported that the hydrocarbon is converted into other hydrocarbons when heated to 200 ° C. A thermolysis C revealed that a ring expansion to the constitutionally isomeric hydrocarbon in the gas phase at 360 to 410 ° methylenecyclobutane takes place; in addition, the fission products ethene and allene are formed . Presumably the longer - weaker - bond in spiropentane is cleaved preferentially; a diradical can be assumed to be an intermediate.
Individual evidence
- ↑ a b c N. Zelinsky, On spirocyclane, its synthesis and its behavior in reduction catalysis . In: Reports of the German Chemical Society , Vol. 46 (1913), pp. 160–172. doi : 10.1002 / cber.19130460128
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-462.
- ↑ a b D. E. Applequist, GF Fanta, BW Henrikson, Chemistry of Spiropentane. I. An Improved Synthesis of Spiropentane . In: Journal of Organic Chemistry , Vol. 23, No. 11, (1958) 1715-1716.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ G. Gustavson, On Vinyltrimethylene . In: Journal for practical chemistry . Volume 54, (1896), pp. 97-104.
- ↑ H.Fecht, About Spirocyclane . In: Reports of the German Chemical Society , Vol. 40 (1907), pp. 3883-3891. doi : 10.1002 / cber.190704003194
- ↑ Literature in D. Wendisch, Carbocyclische Dreiringverbindungen , p. 37. In: Eugen Müller (Ed.), Methods of Organic Chemistry (Houbel-Weyl) , Vol. IV / 3, Thieme, Stuttgart, 1971.
- ↑ G. Dallinga, RK van der Draai, LH Toneman, Recueil des Travaux Chimiques des Pays-Bas 87, 897 (1968).
- ↑ a b J. J. Gajewski, LT Burka, Journal of the American Chemical Society 94, No. 25, 8857 (1972).
- ↑ MC Flowers, HM Frey, Journal of the Chemical Society , 1961, 5550.


