Propadien
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
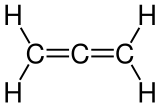
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Propadien | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 4 | |||||||||||||||
| Brief description |
colorless gas |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 40.06 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| Melting point |
−136 ° C |
|||||||||||||||
| boiling point |
−34 ° C |
|||||||||||||||
| Vapor pressure |
0.91 M Pa (21 ° C) |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
190.5 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Propadiene [ … diˈeːn ] is the 1,2- diene of propane and thus the parent compound of the allenes . The common name of the connection is therefore "Allen" . It has the molecular formula C 3 H 4 . The terminal CH 2 groups are in one plane with the middle C atom, but are rotated by 90 ° with respect to one another. Chemical compounds in which one or more hydrogen atoms of propadiene have been exchanged for other radicals can be chiral ( axial chirality ).
Extraction and presentation
The preparation succeeds in good yields in a synthesis sequence starting from allyl bromide , which is first converted to 1,2,3-tribromopropane by bromine addition. Subsequent dehydrohalogenation to 2,3-dibromopropene and reduction using zinc powder gives the propadiene.
properties
In propadiene, the middle carbon atom is sp-hybridized, the two outer carbon atoms are sp 2 - hybridized . One speaks here of cumulative double bonds .
Although propadiene, like its related propene, has double bonds, it does not display the typical chemical and physical properties of alkenes . For example, in the presence of basic catalysts, isomerization to propyne takes place .
- H 2 C = C = CH 2 ⇌ H 3 C − C≡CH
use
Propadiene is used in a mixture with propyne as welding and cutting gas ( MAPP gas ). In organic synthesis, it is used as a raw material in the manufacture of insecticides .
Web links
Individual evidence
- ↑ a b c d entry on all. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ a b c Allene data sheet at Sigma-Aldrich , accessed on April 22, 2011 ( PDF ).
- ↑ a b Entry on all in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-23.
- ↑ Hauptmann / Graefe / Remane: Textbook of Organic Chemistry , Deutscher Verlag der Grundstoffindustrie, Leipzig, 1980, p. 230.
- ^ Norbert Krause (editor), A. Stephen K. Hashmi (editor): Modern Allene Chemistry , Wiley-VCH Verlag, 2004. ISBN 978-3-527-30671-8 .


