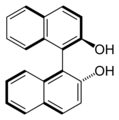
Axial chirality
( R ) - BINOL : The axis of chirality corresponds to the bond between the two naphthyl systems
Axial chirality is a special case of chirality in which the molecule does not have a chiral center but a chiral axis.
Occurrence
Axial chirality occurs in atropisomeric biaryl systems whose rotation around the aryl-aryl bond is prevented, as well as in dihydroanthracenone compounds. The phenomenon was first detected in 1922 for 6,6'-dinitrodiphenic acid.
Furthermore, allene compounds and cumulenes with an even number of cumulative double bonds have axial chirality in the case of asymmetrical substitution (e.g. propadiene derivatives). Spiro compounds can also have axial chirality.
Some trans -cycloalkenes may be axially-chiral, for example, there are of trans - cyclooctene two enantiomers . However, the classification of trans -cycloalkenes as axially chiral is controversial.
Helical molecules such as the helicenes are axially chiral. Due to steric hindrance , the condensed benzene rings are arranged helically around an axis and cannot merge into their mirror image. This type of axial chirality is also called helicity.
An example of the axial chirality in saturated, polycyclic hydrocarbons is trinorbornane .
The enantiomers of axially chiral compounds are identified with the stereochemical descriptors ( R ) and ( S ), in more complicated cases also R a and S a , where the index “a” stands for “axial”. The plus ( P ) / minus ( M ) notation is also used.
application
Axially chiral ligands with C 2 symmetry such as BINAP [2,2'-bis (diphenylphosphino) -1,1'-binaphthyl] are used in enantioselective homogeneous catalysis . The steric and electronic effects can be varied by using suitable ring substituents.
literature
- Eberhard Breitmaier, Günther Jung: Organic chemistry. Thieme Verlag, Stuttgart, 2005. ISBN 3135415058 . ( Limited preview in Google Book search), p. 249.
Web links
- Axial chirality in 6,6'-dinitrobiphenyl-2,2'-dicarboxylic acid, 3D presentation (PDF file; 70 kB) ( Memento from November 23, 2008 in the Internet Archive )
Individual evidence
- ↑ Absolute stereochemistry of fungal metabolites: Icterinoidins A1 and B1, and atrovirins B1 and B2. Melvyn Gill and Peter M. Morgan Arkivoc (RI-1154C) 2004 online article .
- ↑ J. Kenner, WV Stubbings; A Second Form of 6,6'-Dinitrophenic Acid and its conversion into New Cyclic Systems, Journal of the Chemical Society 119 (1921) 593.
- ↑ Bernhard Testa: Fundamentals of Organic Stereochemistry , Verlag Chemie, Weinheim, 1983, pp. 59–61, ISBN 3-527-25935-X .
- ↑ Bernhard Testa: Fundamentals of Organic Stereochemistry , Verlag Chemie, Weinheim, 1983, pp. 64–65, ISBN 3-527-25935-X .
- ↑ Ernest L. Eliel, Samuel H. Wilen: Stereochemistry of Organic Compounds , John Wiles & Sons, 1994, pp. 1172-1175, ISBN 0-471-05446-1 .
- ↑ entry to axial chirality . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.A00547 Version: 2.1.5.
- ^ R. Noyori, Nobel Prize Lecture, December 8, 2001 .
- ^ PJ Walsh, MC Kozlowski: Fundamentals of asymmetric catalysis. University Science Verlag, p. 614 ( excerpt from Google book search).