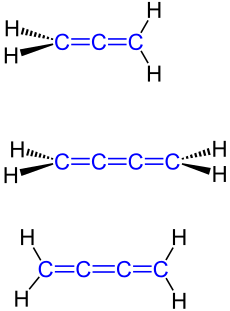
Accumulations
Cumulenes , often cumulenes , (of lat. : Cumulare , pile up ', emphasis on the third syllable: accumulation e ne ) are a group of substances of organic chemical compounds in which the double bonds which are thus directly lined up a system of cumulated double bonds contained.
Whether two cumulative double bonds within a compound are sufficient, as in the allenes , to be added to the group of substances of the cumulenes, is controversially discussed in the specialist literature. The Römpp affirms this, the Gold Book, on the other hand, defines the cumulens as compounds with a sequence of at least three cumulative double bonds and thus excludes the allenes from the cumulens.
Depending on these different definitions, the simplest representatives of the cumulenes are propadiene ("Allen", H 2 C = C = CH 2 ) or butatriene (H 2 C = C = C = CH 2 ).
Compounds which have heteroatoms such as nitrogen , oxygen or sulfur at one or more positions in the double bond system are among the heterocumulenes .
properties
The more cumulative double bonds a cumulus contains, the more its color deepens, similar to that of conjugated polyenes. The cumulenes react very easily with bromine, hydrogen bromide and ozone, but they are relatively resistant to oxygen.
Isomerism
At the two terminal sp 2 -hybridized carbon atoms, differently substituted cumulenes with an odd number of cumulated double bonds form cis - trans isomers . On the other hand, differently substituted cumulenes with an even number of cumulated double bonds at the two terminal sp 2 -hybridized carbon atoms are axially chiral and form enantiomers .
Occurrence
The structural motif of the cumulenes can be found in some natural substances , especially in mushrooms and algae.
use
Heterocumulenes (cumulenes with heteroatoms , e.g. carbodiimides , isocyanates , ketenes ) are mainly used in the laboratory and in technology .
Individual evidence
- ↑ Ivan Ernest: Binding, Structure and Reaction Mechanisms in Organic Chemistry , Springer-Verlag, 1972, ISBN 3-211-81060-9 , p. 28.
- ↑ a b c Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 3: H-L. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1983, ISBN 3-440-04513-7 , p. 2268.
- ↑ Entry on Cumulenes . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C01440 Version: 2.1.5.
- ↑ Bernhard Testa: Fundamentals of Organic Stereochemistry , Verlag Chemie, Weinheim, 1983, ISBN 3-527-25935-X , pp. 72–73.