Ketenes
| Ketenes |
 General structure |
|
Structure of the parent compound ketene |
Ketenes (stress on the second syllable: Ket e ne ) are a group of organic compounds with the general formula RR'C = C = O. R and R 'can be any radicals . Ethenone (= ketene) is the simplest representative of this group of substances with the formula H 2 C = C = O. Ketenes are very reactive, almost all of them dimerize immediately to the corresponding diketene . Ketenes are formally internal anhydrides of the corresponding carboxylic acids.
history
Ketenes were first discovered by Hermann Staudinger in 1905 in the form of diphenyl ketene (conversion of -chlorodiphenylacetyl chloride with zinc) and systematically investigated. He was inspired by the first examples of reactive organic intermediates and stable radicals that Moses Gomberg discovered in 1900 (compounds with a triphenylmethyl group ). With the ketenes, Staudinger found a new such class of substances.
Manufacturing
Ketene is produced industrially by catalytic high-temperature pyrolysis of acetone or acetic acid at 700 ° C.
- Ketene synthesis by pyrolysis of acetone. Methane is produced as a by-product.
In general, ketenes are accessible in situ by reacting carboxylic acid halides , which carry at least one hydrogen in the α position, with bases such as triethylamine :
Ketenes can also result from a Wolff rearrangement .
Reactions
Because of their accumulated double bonds, ketenes are very reactive.
Formation of carboxylic acid esters
Carboxylic acid esters are formed by reaction with alcohols :
Formation of carboxylic acid anhydride
A ketene reacts with a carboxylic acid to form a carboxylic acid anhydride :
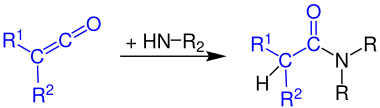
Formation of carboxamides
Ketenes react with ammonia to form primary amides :
The reaction of ketenes with primary amines produces secondary amides:
Ketenes react with secondary amines to form tertiary amides:
hydrolysis
Carboxylic acids are formed from ketenes by reaction with water:
Formation of enol acetates
With enolizable carbonyl compounds , ketenes are converted into enol acetates. The following example shows the reaction of ethenone with acetone to form propen-2-yl acetate:
Dimerization
At room temperature , ketene rapidly dimerizes to diketene , but it can be recovered by heating:
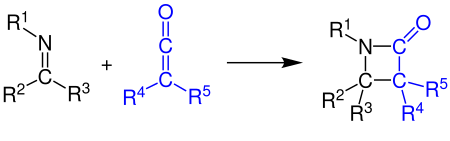
[2 + 2] cycloaddition
Ketenes can u. a. react with alkenes , carbonyl compounds , carbodiimides and with imines under [2 + 2] cycloaddition . The example shows the synthesis of a β-lactam by the reaction of a ketene with an imine (see Staudinger synthesis ):
Individual evidence
- ↑ Staudinger, Ketene, a new body class . Reports of the German Chemical Society, Volume 38, 1905, pp. 1735-1739.
- ↑ Thomas T. Tidwell, The first century of Ketenes (1905-2005): the birth of a family of reactive intermediates, Angewandte Chemie, Int. Edition, Volume 44, 2005, pp. 5778-5785.
- ^ Siegfried Hauptmann : Organic Chemistry, 2nd Edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 410, ISBN 3-342-00280-8 .
- ^ Siegfried Hauptmann: Organic chemistry: with 65 tables . German publishing house for basic industry, Leipzig 1985, ISBN 3-87144-902-4 , p. 410-412 .
- ^ Jie Jack Li: Name reactions. A collection of detailed reaction mechanisms . 3. Edition. Springer-Verlag, Berlin 2006, ISBN 978-3-540-30030-4 , pp. 561-562 , doi : 10.1007 / 3-540-30031-7 .
- ^ Hermann Staudinger: On the knowledge of the ketenes. Diphenylketene . In: Justus Liebig's Annals of Chemistry . tape 356 , no. 1-2 . John Wiley & Sons, Inc., 1907, p. 51-123 , doi : 10.1002 / jlac.19073560106 .