Diphenylketene
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Diphenylketene | ||||||||||||
| other names |
2,2-diphenylethenone |
||||||||||||
| Molecular formula | C 14 H 10 O | ||||||||||||
| Brief description |
orange oil |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 194.23 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| density | |||||||||||||
| Melting point |
8-9 ° C |
||||||||||||
| boiling point |
|
||||||||||||
| solubility |
soluble in benzene and tetrahydrofuran |
||||||||||||
| Refractive index |
1.615 (20 ° C, 589 nm) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||
Diphenylketene was first isolated by Hermann Staudinger in 1905 and identified as the first representative of the extremely reactive class of ketenes with the general formula R 1 R 2 C = C = O (R 1 = R 2 = phenyl group ). With the cumulative double bonds in the ketene structure R 1 R 2 C = C = O, diphenyl ketene represents a heterocumulene . The most important reaction of diphenyl ketene is the [2 + 2] cycloaddition on CC, CN, CO, CS multiple bonds.
Occurrence and representation
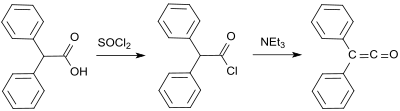
The first synthesis by H. Staudinger was based on chlorodiphenyl acetyl chloride (from benzilic acid and thionyl chloride ), from which two chlorine atoms are split off with zinc in a dehalogenation reaction .
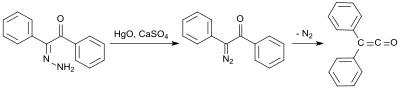
An early synthesis uses benzil monohydrazone (from benzil and hydrazine hydrate ), which is oxidized with mercury (II) oxide and calcium sulfate to form the monodiazoketone and then rearranged at 100 ° C with nitrogen elimination in 58% yield to the diphenylketene.
Another early diphenyl ketene synthesis comes from Eduard Wedekind , who obtained diphenyl ketene from the dehydrohalogenation of diphenylacetyl chloride with triethylamine in 1901 , without isolating and characterizing it. This variant was also described by H. Staudinger in 1911.
A laboratory standard is based on the Staudinger process and delivers diphenylketene as an orange-colored oil in yields of 53 to 57%.
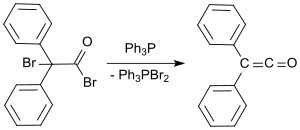
In a more recent process, 2-bromo-2,2-diphenylacetyl bromide is reacted with triphenylphosphine to give diphenylketene in yields of up to 81%.
A synthesis of diphenylketene from diphenylacetic acid and the Hendrickson reagent (triphenylphosphonium anhydride triflate, from triphenylphosphine oxide and trifluoromethanesulphonic anhydride ) with elimination of water in 72% yield has recently been reported.
properties
Diphenylketene is an orange to red oil at room temperature - with the color of concentrated potassium dichromate solution - that reacts with non-polar organic solvents such as. B. diethyl ether, acetone , benzene, tetrahydrofuran, chloroform mixes and solidifies in the cold to yellow crystals. The compound is easily oxidized by air, but can be stored in tightly closed containers at 0 ° C for several weeks without decomposition or in a nitrogen atmosphere with the addition of a small amount of hydroquinone as a polymerization inhibitor.
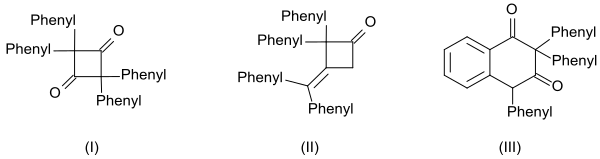
The high reactivity of the diphenyl ketene is also manifested in the formation of three defined dimers:
- the cyclic diketone 2,2,4,4-tetraphenylcyclobutane-1,3-dione (I) by heating with quinoline
- the β-lactone 4- (diphenylmethylene) -3,3-diphenyloxetan-2-one (II) by heating with sodium methoxide and
- the tetralin derivative 2,2,4-triphenylnaphthalene-1,3- (2 H , 4 H ) -dione (III) by heating with benzoyl chloride
and higher oligomers derived therefrom .
Applications
In their constitution and in their reactivity, ketenes of the general formula R 1 R 2 C = C = O show many parallels to isocyanates of the general formula RN = C = O.
Diphenylketene adds water to form diphenylacetic acid, ethanol to form ethyl diphenylacetate, or ammonia to form the corresponding amide. Mixed anhydrides of diphenylacetic acid are formed with carboxylic acids, which can be used to activate protected amino acids for peptide linkage.
Activating Z - leucine with diphenyl ketene and subsequent reaction with phenylalanine ethyl ester produces the protected dipeptide Z-Leu-Phe-OEt (N-benzyloxycarbonyl-L-leucyl-L-phenylalanine ethyl ester) in 59% yield .
Diphenylketene prone to autoxidation , located in an intermediate arising diphenyl acetolactone forms at temperatures above 60 ° C, the corresponding polyester.
In a Wittig reaction , allenes can be prepared from diphenylketene .
With triphenylphosphinediphenylmethylene and diphenylketene z. B. at 140 ° C under pressure tetraphenylallene in 70% yield.
The synthetically most interesting reactions of the diphenyl ketene are [2 + 2] cycloadditions, such as B. the reaction with cyclopentadiene to form a Diels-Alder adduct .
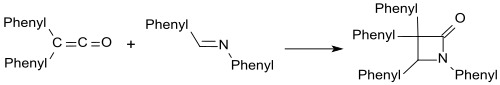
Imines such as B. Benzalaniline form β- lactams with diphenylketene
and with carbonyl compounds, β- lactones are formed analogously .
The [2 + 2] cycloaddition of diphenyl ketene with phenylacetylene first leads to a cyclobutenone, which is thermally aromatized to a phenyl vinyl ketene and cyclized in a [4 + 2] cycloaddition to 3,4-diphenyl-1-naphthol in 81% yield .
A general synthetic method for substituted phenols and quinones has developed from this so-called Smith-Hoehn reaction.
Individual evidence
- ↑ a b c E.C. Taylor, A. McKillop, GH Hawks: Diphenylketene In: Organic Syntheses . 52, 1972, p. 36, doi : 10.15227 / orgsyn.052.0036 ; Coll. Vol. 6, 1988, p. 549 ( PDF ).
- ↑ a b E. Lax: D'Ans-Lax, pocket book for chemists and physicists, 3rd edition Springer, Berlin 1964, ISBN 978-3-642-49526-7 , p. 2-418 .
- ↑ a b S.D. Darling, RL Kidwell: Diphenylketene. Triphenylphosphine dehalogenation of .alpha.-bromodiphenylacetyl bromide . In: J. Org. Chem. Band 33 , no. 10 , 1968, p. 3974-3975 , doi : 10.1021 / jo01274a074 .
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, 2016, ISBN 978-1-4987-5429-3 , pp. 230 ( limited preview in Google Book search).
- ↑ a b c d e f H. Staudinger: Ketene, a new body class . In: Ber. German Chem. Ges. Volume 38 , no. 2 , 1905, p. 1735-1739 , doi : 10.1002 / cber.19050380283 .
- ↑ a b c J.W. Leahy: Diphenylketene . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2001, doi : 10.1002 / 047084289X.rd421 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b T.T. Tidwell: The first century of ketenes (1905-2005): The birth of a versatile family of reactive intermediates . In: Angew. Chem. Band 44 , no. 36 , 2005, pp. 5778-5785 , doi : 10.1002 / anie.200500098 .
- ↑ H. Ulrich: Cycloaddition Reactions of Heterocumulenes . Academic Press, New York 1967, pp. 374 .
- ^ FE King, D. Holmes: Synthetic mydriatics. Diphenylchloroacetyl chloride as a reagent for the preparation of benzylic esters of tertiary amino-alcohols . In: J. Chem. Soc. 1947, p. 164-168 , doi : 10.1039 / JR9470000164 .
- ↑ T. Curtius, K. Thun: Effect of hydrazine hydrate on monoketones and orthodiketones . In: J. Prakt. Chem. Band 44 , no. 2 , 1891, p. 161-186 , doi : 10.1002 / prac.18910440121 .
- ↑ a b L.I. Smith, HH Hoehn: Diphenylketene In: Organic Syntheses . 20, 1940, p. 47, doi : 10.15227 / orgsyn.020.0047 ; Coll. Vol. 3, 1955, p. 356 ( PDF ).
- ↑ E. Wedekind: About the production of acid anhydrides with the help of tertiary amines . In: Ber. German Chem. Ges. Volume 34 , no. 2 , 1901, p. 2070-2077 , doi : 10.1002 / cber.190103402122 .
- ↑ H. Staudinger: About Ketene.XIX. About the formation and representation of the diphenyl ketene . In: Ber. German Chem. Ges. Volume 44 , no. 2 , 1911, p. 1619–1623 , doi : 10.1002 / cber.19110440258 .
- ↑ Simon Nuß: Synthesis of new ring-A-modified derivatives of the alkaloid Luotonin A. (PDF; 2.9 MB) University of Vienna , 2012, p. 16 , accessed on April 2, 2019 (diploma thesis).
- ↑ JI McCauley: Hendrickson reagent (triphenylphosphonium anhydride trifluoromethane sulfonate) . In: Synlett . tape 23 , no. 20 , 2012, p. 2999-3000 , doi : 10.1055 / s-0032-1317486 .
- ^ Z. Moussa: The Hendrickson 'POP' reagent and analogues thereof: synthesis, structure, and application in organic synthesis . In: ARKIVOC . i, 2012, p. 432-490 ( arkat-usa.org ).
- ↑ H. Das, EC Kooyman: Oligomers of diphenylketene . In: Recl. Trav. Chim. Pays-Bas . tape 84 , no. 8 , 1965, p. 965-978 , doi : 10.1002 / rec.19650840802 .
- ↑ G. Losse, E. Demuth: Diphenylketene as a reagent for the formation of peptide bonds . In: Ber. German Chem. Ges. Volume 94 , no. 7 , 1961, pp. 1762–1766 , doi : 10.1002 / cber.19610940713 .
- ↑ H. Staudinger, K. Dyckerhoff, HW Klever, L. Ruzicka: About autoxidation of organic compounds. IV .: About autoxidation of ketenes . In: Ber. German Chem. Ges. Volume 58 , no. 6 , 1925, pp. 1079-1087 , doi : 10.1002 / cber.19250580618 .
- ↑ G. Wittig, A. Haag: About phosphine-alkylenes as olefin-forming reagents, VIII. Allele derivatives from ketenes . In: Ber. German Chem. Ges. Volume 96 , no. 6 , 1963, pp. 1535-1543 , doi : 10.1002 / cber.19630960609 .
- ↑ G. Lüscher: Contribution to the constitution of the aliphatic diazo bodies and hydrazones. New organic phosphorus compounds . Ed .: Swiss Federal Institute of Technology. Zurich 1922, doi : 10.3929 / ethz-a-000096667 ( e-collection.library.ethz.ch [PDF]).
- ↑ a b H. Staudinger: On the knowledge of the ketenes. Diphenylketene . In: Liebigs Ann. Chem. Band 356 , no. 1-2 , 1907, pp. 51-123 , doi : 10.1002 / jlac.19073560106 .
- ↑ LI Smith, HH Hoehn: The reaction of diphenylketene and phenylacetylene . In: J. Am. Chem. Soc. tape 61 , no. 10 , 1939, pp. 2619–2624 , doi : 10.1021 / ja01265a015 .