Tetrahydronaphthalene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetrahydronaphthalene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 12 | |||||||||||||||
| Brief description |
colorless liquid with a naphthalene odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 132.20 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.97 g cm −3 |
|||||||||||||||
| Melting point |
−36 ° C |
|||||||||||||||
| boiling point |
208 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.5414 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 2 ml m −3 or 11 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Tetrahydronaphthalene ( 1,2,3,4-tetrahydronaphthalene , IUPAC ) is a hydrocarbon with the empirical formula C 10 H 12 . The historical name Tetralin is still frequently used today, but has been a registered word mark of Dehydag , now Cognis, since 1956 .
Manufacture and extraction
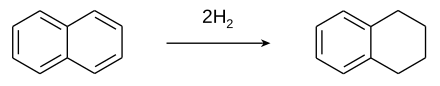
Tetrahydronaphthalene is made by the partial catalytic hydrogenation of naphthalene . Decalin (decahydronaphthalene) is obtained from it through complete hydrogenation .
properties
The colorless, oily liquid solidifies at approximately −36 ° C and boils at 208 ° C. The odor of tetrahydronaphthalene is similar to that of naphthalene . The liquid is highly refractive, the refractive index is 1.541. Tetrahydronaphthalene is almost insoluble in water; it floats on water because its density of 0.973 g · cm −3 is lower than that of water. It is readily soluble in most organic solvents. With a water content of 80 mol%, an azeotropic boiling point of 99 ° C. is observed at atmospheric pressure .
The compound forms inflammable vapor-air mixtures above its flash point at 71 ° C. The explosion range is between 0.5% by volume (45 g / m³) as the lower explosion limit (LEL) and 5.0% by volume (275 g / m³) as the upper explosion limit (UEL). The ignition temperature is 390 ° C. The substance therefore falls into temperature class T2.
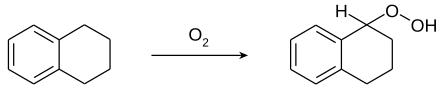
Tetrahydronaphthalene easily forms 1-hydroperoxy-tetrahydronaphthalene ("tetralin hydroperoxide") with atmospheric oxygen. This must be taken into account when distilling older products, as explosions may occur.
use
Tetrahydronaphthalene is used as a solvent for rubbers , paint remover and heat exchange fluid. It is found in paints, paint strippers, and pesticides (as a solvent). Tetrahydronaphthalene is used in the production of 1-naphthol , detergents, plasticizers, shoe polishes, floor polishes and textile auxiliaries.
Isomeric compounds
Of the various theoretically possible tetrahydronaphthalene derivatives, 1,4,5,8-tetrahydronaphthalene ( 2 ) is accessible via a Birch reduction of naphthalene ( 1 ). 1,4,5,8-Tetrahydronaphthalene is the starting material for various heptalene syntheses.
Individual evidence
- ↑ a b c Entry on tetralin. In: Römpp Online . Georg Thieme Verlag, accessed on March 10, 2014.
- ↑ a b c d e f g h i j k l m Entry on tetrahydronaphthalene in the GESTIS substance database of the IFA , accessed on November 11, 2017(JavaScript required) .
- ↑ Entry on 1,2,3,4-tetrahydronaphthalene in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 119-64-2 or tetrahydronaphthalene ), accessed on November 2, 2015.
- ^ Trademark information from the DPMA : Tetralin , accessed on August 9, 2013.
- ↑ a b Brockhaus ABC Chemie , FA Brockhaus Verlag Leipzig 1971, p. 1390.
- ↑ a b Data sheet 1,2,3,4-Tetrahydronaphthalene, anhydrous, 99% from Sigma-Aldrich , accessed on November 11, 2017 ( PDF ).
- ^ IM Smallwood: Handbook of organic solvent properties , Arnold London 1996, ISBN 0-340-64578-4 , pp. 55-57.
- ^ E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ^ Gerhard Becker, Heinz Kolshorn: Heptalene . In: Heinz Kropf (Ed.): Houben-Weyl Methods of Organic Chemistry . Vol. V / 2c. Georg Thieme Verlag, Stuttgart, New York 1985, ISBN 3-9801412-1-7 , pp. 418 ff . ( limited preview in Google Book search).