Quinoline
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Quinoline | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 7 N | |||||||||||||||
| Brief description |
colorless to yellowish, strongly refractive, hygroscopic liquid, colorless, deliquescent prisms as hydrochloride hydrate |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 129.16 g · mol -1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.10 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
237.2 ° C |
|||||||||||||||
| Vapor pressure |
8 Pa (20 ° C) |
|||||||||||||||
| pK s value |
4.94 (conjugate acid) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Dipole moment |
2.29 D |
|||||||||||||||
| Refractive index |
1.6262 (21 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
141.22 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Quinoline , also known as azanaphthalene or benzo [ b ] pyridine , is an organic compound from the group of heteroaromatic compounds and belongs to the dinuclear heterocyclic parent systems. It consists of two fused aromatic six-membered rings - one benzene - and one pyridine ring - from which the empirical formula C 9 H 7 N results. Formally, it is therefore a naphthalene molecule in which a carbon atom of the ring structure has been replaced by a nitrogen atom . Quinoline is a colorless, water-binding liquid with an unpleasant, pungent odor. As a heteroaromatic, quinoline has a lower reactivity than naphthalene with regard to electrophilic aromatic substitution , but in comparison it is easier to enter into nucleophilic aromatic substitutions .
Quinoline is found in coal tar and can be expelled from coal . There are numerous natural derivatives of quinoline, which are often found as alkaloids in plants (see quinoline alkaloids ). This group includes the China alkaloids, with quinine being the best-known representative. Quinoline was first isolated in pure form from coal tar by Friedlieb Ferdinand Runge in 1834 . In 1842 it was obtained from Charles Frédéric Gerhardt by breaking down the alkaloid cinchonine , from which the name quinoline is derived.
Quinoline is an important raw material in the chemical and pharmaceutical industries . It is used in the manufacture of drugs , herbicides and fungicides and as a basic solvent . Although several synthetic approaches to quinoline are known, for economic reasons a large part of the worldwide demand for quinoline is still covered by insulation from coal tar.
history
The isolation of pure quinoline was first achieved by Friedlieb Ferdinand Runge in 1834. He extracted it from coal tar and named the compound leucoline . Quinoline was 1842 a second time by Charles Frederic Gerhardt discovered that the decomposition products of quinine and cinchonine by the action of alkalis analyzed and supposedly the same in both cases, knowledge by hitherto unknown chemical compound received.
“The following fact is not uninteresting. The quinine, under the influence of caustic potash, is transformed into a new nitrogenous base that is oily at ordinary temperature. This new compound, which I call quinoline ... "
The name was given based on the compounds quinine and cinchonine , from which he had obtained quinoline. In 1843 Gerhardt referred to the compound as quinoleïn , later also quinolein (compare also English quinoline ) was used. Gerhardt, however, erred in assuming that quinoline occurs as a breakdown product of both quinine and cinchonine, because, as was shown later, a methoxylated quinoline derivative results from the breakdown of quinine , whereas only the breakdown of cinchonine yields unsubstituted quinoline. The molecular structures of Runge's Leucoline and Gerhardt's Quinoline were still unknown at the time of discovery. It was not until 1882 that Hoogewerff and van Dorp clarified the identity of the two connections .
«The expériences décrites dans le précédentes semplent démontret qu'il ya dans le goudron de houille une leucoline identique à la quinoléine obtenue de la cinchonine. »
"The test results described on the previous pages seem to indicate that there is a leucoline in coal tar that is identical to the quinoline obtained from cinchonine."
From this point on, the name quinoline began to gain acceptance.
The molecular structure of quinoline was clarified in 1879. It was previously postulated that quinoline was naphthalene, in which one carbon atom in the ring was replaced by nitrogen . Since naphthalene could be prepared a few years earlier by the cyclization of 4-phenyl-1-butene, this hypothesis could be confirmed by the analogous cyclization of N -allylaniline to quinoline. Koenigs succeeded in doing this in 1880 using lead (II) oxide , which allowed the postulated structure of quinoline as a naphthalene analogue to be substantiated.
In the following decades the chemical and physical properties of the compound were clarified in numerous studies and various synthetic routes for quinoline and its derivatives were established.
Occurrence
Quinoline is practically non-free in nature, but forms the basic structure of numerous natural substances from which it can be released through degradation reactions. This includes the group of china alkaloids, which occur in high concentrations in cinchona bark trees . Quinoline found to about 0.3% - along with many other heterocyclic compounds - in the coal tar , a by-product of coke production from coal . Quinoline is analytically detectable in tobacco smoke.
nomenclature
In addition to quinoline , the names benzopyridine and 1-azanaphthalene are occasionally used. Benzopyridine describes the molecule as pyridine with a fused-on benzene ring and is ambiguous without further qualifiers, since this designation also applies to the isomeric isoquinoline ; Benzo [ b ] pyridine is correct and unambiguous . 1-Azanaphthalene describes quinoline as a derivative of naphthalene in which the carbon atom (or rather the methine group) in the 1-position has been replaced by a nitrogen atom. In contrast to benzopyridine , this designation is clear.
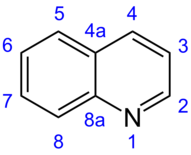
The numbering of the ring atoms follows the general rule for polynuclear aromatic systems. Counting is started on the highest-ranking stem system, which in the present case is the pyridine ring. As a heteroatom, the nitrogen atom is assigned the highest priority and thus the smallest possible number. In the case of quinoline, the nitrogen atom has the number 1 and the carbon ring atoms of the pyridine ring are consecutively numbered 2-4 from there. The counting is continued continuously in the benzene ring, the bridging atoms being skipped. These are assigned the designations 4 a and 8 a according to the general numbering scheme for condensed ring systems .
The systematic name of the quinoline residue is quinolyl , whereby the position of the link is prefixed as a number. Analogous to the pyridine residue ( pyridyl instead of systematically pyridinyl ), the systematic name quinolinyl is rarely used. The fusing component of quinoline as the parent system in condensed polycyclic aromatic systems is chino .
Extraction and presentation
Like a number of other heterocyclic nitrogen bases (for example pyridine and pyrrole ), quinoline can be obtained from coal tar, in which it is 0.3%. In contrast to pyridine, the global demand for which is now largely met by synthetic processes, quinoline is still largely obtained from coal tar. Through fractional distillation , it is converted into the methylnaphthalene fraction together with isoquinoline and quinaldine , from which it is extracted with sulfuric acid together with methylnaphthalene and isoquinoline . The methylnaphthalene is then separated off by precipitation with ammonia . Due to the 6 ° C higher boiling point of isoquinoline, the remaining mixture of quinoline and isoquinoline can be separated by rectification . There are various options for further purification of quinoline, such as the resinification of impurities with formaldehyde , treatment with alkalis, selective oxidation and the formation of hydrates (isoquinoline does not form hydrates). Furthermore, quinoline can also be obtained directly from the methylnaphthalene fraction by azeotropic rectification with ethylene glycol or diethylene glycol and then purified by distillation.
Skraup synthesis
According to Koenig first quinoline dating from 1879, the skraup reaction which by Zdenko Hans Skraup was first published in 1880 the second synthetic access to quinoline. It is based on aniline , which in the presence of glycerol , sulfuric acid and an oxidizing agent to Quinoline is implemented. Glycerin is first dehydrated to acrolein , which is required as an α, β-unsaturated carbonyl compound for cyclization. The direct use of acrolein, on the other hand, reduces the yield, since it tends to polymerize under the reaction conditions . The Skraup synthesis initially provides dihydroquinoline, which can be oxidized to quinoline by mild oxidizing agents such as trivalent iron salts, nitrobenzene or iodine . The Skraup synthesis is one of the few direct synthetic routes to unsubstituted quinoline.
The Doebner-Miller reaction is a modification of the Skraup synthesis and directly uses α, β-unsaturated aldehydes , whereby quinolines substituted in the 2-position can be produced.
Friedländer quinoline synthesis
The Friedländer quinoline synthesis starts from o -aminobenzaldehyde , which is cyclized with enolizable carbonyl compounds to derivatives of quinoline. The reaction is catalyzed by trifluoroacetic acid , toluenesulfonic acid , iodine and various Lewis acids .

The Niemantowski synthesis is a modification of the Friedländer quinoline synthesis, which starts from anthranilic acid instead of o- aminobenzaldehydes .
Further synthesis routes
A number of other synthetic routes to quinoline or its derivatives are known. The Conrad-Limpach synthesis (according to Max Conrad , Leonhard Limpach ), in which anilines and β-ketoesters are used, and the Povarov reaction , for which aniline, benzaldehyde and activated alkenes are required, should be mentioned by name. The Camps quinoline synthesis , the Knorr quinoline synthesis and the Gould-Jacobs reaction are also used.
Some quinoline alkaloids occur as natural substances in biological systems. The exact biosynthetic structure of quinoline depends on the biological system and the exact structure of the quinoline derivative. The biochemical access of some quinoline derivatives is the amino acid tryptophan , from which the quinoline structure can be built up in a multi-stage reaction. Via a further synthetic route starting from anthranilic are hydroxylated Chinolingerüste accessible. The continuation of the synthesis leads to acridine derivatives.
properties
Physical Properties
Quinoline is colorless and liquid under standard conditions. It boils at 237.2 ° C and freezes at -14.8 ° C. According to Antoine, the vapor pressure function results according to log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 3.94043, B = 1667.104 and C = −87.085 in the temperature range from 437.8 to 511.1 K. It is a highly light-refractive liquid that has a refractive index of 1.6262 at 21 ° C and a wavelength of 589 nm . Under standard conditions, quinoline has a density of 1.10 g · cm −3, comparable to that of water . Quinoline is diamagnetic (the molar diamagnetic susceptibility is -86.1 x 10 -6 cm 3 · mol -1 ) and has a dipole moment of 2.29 D to. The critical temperature is 527 ° C, the critical pressure 57.8 bar. In the liquid phase the standard enthalpy of formation is 141.22 kJ mol −1 , whereas in the gas phase it is 200.5 kJ mol −1 . At 25 ° C, quinoline has a viscosity of 3.337 mPa · s −1 and a thermal conductivity of 0.147 W · (m · K) −1 .
As a solid , the compound occurs in two polymorphic crystal forms. The conversion from crystal form II to crystal form I takes place at −53 ° C. The crystal form II crystallizes in the monoclinic crystal system in the space group P 2 1 / c (space group number 14) with the lattice parameters a = 992 pm , b = 1085 pm, c = 1337 pm and determined at 150 K (−123 ° C) β = 106.5 ° and eight formula units per unit cell . The individual molecules are arranged in two orthogonal chains that are held together by weak CHN hydrogen bonds . Between the chains, there are interactions between C – H bonds and the aromatic π system.
Quinoline is only sparingly soluble in water. At 20 ° C, only 6 g per liter dissolve. Its significantly poorer solubility compared to pyridine, which is freely miscible with water , is due to the non-polar benzene ring. In contrast, quinoline is freely miscible with ethanol , diethyl ether , acetone , benzene and carbon disulfide .
Chemical properties
Quinoline has a weakly basic reaction and, in the presence of hydrochloric acid, forms a crystalline hydrochloride (C 9 H 7 N · HCl), which melts at 134 ° C.
Quinoline belongs to the group of heteroaromatic compounds, the properties of which are reflected in its reactivity. Compared to its carbon analogue naphthalene, it is less reactive with regard to electrophilic substitutions , which is due to the electron-withdrawing properties of the nitrogen atom, which on the one hand reduces the electron density in the aromatic system and on the other hand is able to react with attacking electrophiles to form quinolinium compounds that are even more electron poor. In contrast to naphthalene, however, quinoline has a comparatively higher reactivity with regard to nucleophilic substitutions . The nitrogen atom is sp 2 -hybridized and has typical basic properties of an amine . Electrophilic aromatic substitutions tend to take place on the benzene ring, while nucleophilic aromatic substitutions tend to take place on the pyridine ring.
Similar to pyridine, the reaction with many Lewis acids leads to addition to the nitrogen atom.
Molecular Properties
Quinoline has a fully conjugated system with ten π electrons , which are delocalized over the entire ring system . The heteroaromatic molecule has a planar structure, but the electron density is not evenly distributed, which is due to the negative inductive effect of the nitrogen atom. For this reason, quinoline, like pyridine, has a dipole moment.
The bonds in the molecule have different lengths . As a ligand in the nickel complex, they are between 133 and 145 pm and, as usual for aromatic systems, are between the values that are typically expected for single-bonded and double-bonded atoms. In comparison, naphthalene has C – C bond lengths between 135 and 142 pm, which suggests a more uniform electron distribution in this molecule.
All ring atoms in the quinoline molecule are sp 2 - hybridized . The nitrogen atom provides the electron of its p orbital for the formation of the aromatic system, its free sp 2 electron pair lies in the molecular plane and points away from the ring center. Due to its position, it cannot interact with the π system and therefore does not contribute to the development of aromaticity. However, it is important for the chemical properties of quinoline because, unlike naphthalene, the aromatic system is not neutralized by the addition of an electrophile at this position. The separation of the lone pair of electrons from the aromatic system also means that the nitrogen atom cannot develop a positive mesomeric effect . The reactivity of the pyridine ring in the quinoline molecule is largely determined by the negative inductive effect of the nitrogen atom. However, its influence is less in the benzene ring.
Quinoline is resonance-stabilized via seven mesomeric boundary structures. Similar to naphthalene, there are two boundary structures that do not have a zwitterionic character. In addition, however, five other zwitterionic boundary structures can be formulated, which assign a negative charge to the nitrogen atom , whereby the positive charge is distributed over the aromatic system. The position of the charge on the nitrogen atom is consistent with its higher electronegativity compared to carbon.
Reactions
Electrophilic substitutions
In comparison to pyridine, quinoline reacts more easily in the sense of an electrophilic aromatic substitution. This fact is due to the higher average electron density in the aromatic system, which is caused by the relatively electron-rich benzene ring of the compound. Due to the higher electron density in the benzene ring, electrophilic substitutions take place preferentially on this. Often Brønsted or Lewis acids are also present in the reaction mixture for electrophilic substitutions . These can add to the nitrogen atom of the pyridine ring and thus cause an even stronger deactivation of the pyridine ring. For the electronic reasons described, electrophilic substitutions occur fastest at the 5- and 8-positions of the quinoline. A mixture of equal parts of 5- and 8-nitroquinoline is obtained as the nitration product , while other isomers are only formed to a minor extent. The nitration of quinoline has a weaker selectivity than that of isoquinoline, in which almost exclusively 5-nitroisoquinoline is formed.
By sulfonation with oleum , the in-8 position is substituted quinoline derivative as the main product, and further, the 5-Chinolylderivat obtained at moderate temperature. Since the electrophilic substitution takes place the fastest at these positions, these are the kinetic reaction products. When the product mixture is heated to above 250 ° C., isomerization to the thermodynamically more favorable quinoline-6-sulfonic acid takes place.
The composition of the product spectrum obtained by halogenating quinoline with molecular halogens is strongly subject to the reaction conditions. By bromination in sulfuric acid , however, the quinoline derivatives substituted in the 5- and 8-position are usually the main products of the reaction. By using the quinoline hydrobromide, substitution on the pyridine ring is also possible under mild reaction conditions, which takes place in the 3-position.
Nucleophilic Substitutions

Many nucleophilic substitutions known from pyridine chemistry also take place on quinoline, preferably at the electron-poor 2-position of the pyridine ring. This includes amination by a Chichibabin reaction , in which the amide ion preferably adds to the 2-position of quinoline by using potassium amide as a nucleophile in liquid ammonia at -66 ° C. Subsequent oxidation with potassium permanganate can release 2-aminoquinoline . When the reaction temperature is increased to −40 ° C, isomerization to the thermodynamically more stable 4-substituted product takes place.
Quinoline can often be directly alkylated or arylated by using the underlying lithium organyls . The dihydroquinoline derivative formed as an intermediate product after aqueous work-up of the reaction can be thermally rearomatized . If good leaving groups are present, a number of ipso substitutions on quinoline are known , analogous to pyridine . Substitutions at the 3-position tend to have characteristics of ipso -substitutions on the corresponding halogen aromatic compounds , while those at the 2- and 4-positions are similar to those on pyridine.
Lithiated quinolines can be produced from the halogen derivatives on which they are based, both on the pyridine and on the benzene ring, using commercially available lithium organyls such as n -butyllithium . This reaction competes with the alkylation described above, which, however, can be largely suppressed in many cases by carrying out the reaction at low temperatures. The lithiated quinoline derivatives obtained can either be used directly as nucleophiles or previously transmetalated to another metal ion .
Oxidation and reduction
Analogous to the pyridine N oxide and quinoline forms an N oxide obtained by oxidation of quinoline with peroxycarboxylic acids , often perbenzoic acid can be prepared. In contrast, under strongly oxidizing conditions, the oxidative degradation of the benzene or pyridine ring occurs. Which of the rings is subject to degradation depends on the reaction conditions. As a rule, the benzene ring is affected, which often results in quinolinic acid. Potassium permanganate , manganese dioxide or fuming nitric acid can serve as oxidizing agents . However, the most efficient method is electrochemical oxidation. The ozonolysis of quinoline yields pyridine-2,3-dialdehyde , which can in turn be oxidized to quinolinic acid by subsequent oxidation with hydrogen peroxide .
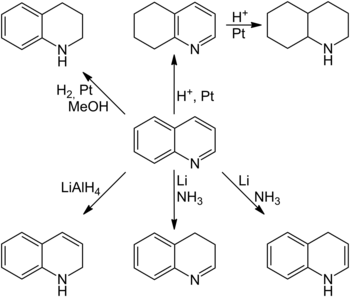
Depending on the reaction conditions, both the benzene and the pyridine ring can be selectively hydrogenated . Classical molecular hydrogen in methanol at room temperature on a platinum catalyst is used to hydrogenate the pyridine ring . However, hydrogenations using sodium cyanoborohydride and sodium borohydride and zinc borohydride in the presence of nickel (II) chloride are also known. The selective hydrogenation of the benzene ring is achieved by reaction with hydrogen in strong acids over a platinum catalyst . The fully saturated decahydroquinoline can also be obtained under these conditions through a longer reaction time. Dihydrogenated quinoline derivatives are also synthetically accessible from quinoline. 1,2-Dihydroquinoline can be produced by reduction with lithium aluminum hydride , while 1,4- and 3,4-dihydroquinoline are accessible by reduction with elemental lithium in liquid ammonia. However, dihydrogenated quinoline derivatives often tend to isomerization of the double bond and can therefore sometimes not be isolated, but only occur as intermediate products of a reaction.
use
Ullmann's Encyclopedia of Technical Chemistry puts the world annual production of quinoline at 2000 tons in 2005 . In analogy to the increasing demand for similar heterocyclic synthetic building blocks, however, it can be assumed that the production capacity has meanwhile increased. Quinoline has a wide range of uses in the chemical industry. It is an important raw material for the manufacture of medicines as well as herbicides and fungicides . It is also used as a basic catalyst, for example in the pharmaceutical industry .
Some of the main uses of quinoline include making 8-hydroxyquinoline , a complexing agent used as a disinfectant and antifungal agent . The synthesis of 8-hydroxyquinoline is achieved by sulfonating quinoline to quinoline-8-sulfonic acid and then ipso - hydroxylation with hot sodium hydroxide solution . Furthermore, quinoline is used to produce quinolinic acid , which is an important raw material for the production of herbicides such as imazapyr . Quinoline is also a raw material for the industrial synthesis of nicotinic acid (vitamin B 3 ), which can be obtained by decarboxylating quinolinic acid. The classic degradation is oxidation using strong oxidizing agents such as potassium permanganate , but modern industrial processes use cheaper oxidizing agents.

Partially hydrogenated quinoline derivatives are also used in the production of active pharmaceutical ingredients and antibiotics . 2-Hydroxyquinoline , which can be obtained oxidatively by hypochlorous acid or enzymatic hydroxylation from quinoline, is also a raw material for the production of, for example, cardiac drugs or antihistamines . It is also the raw material for the industrial synthesis of cyanine dyes .
In chemical laboratories or chemical plants, quinoline can be used as a good solvent and very good extractant for polycyclic aromatic hydrocarbons . Sometimes mixtures with isoquinoline are used for this purpose. Quinoline is also used as a corrosion inhibitor and as an acid-binding base in chemical reactions. In the palladium- catalyzed hydrogenation of alkynes , quinoline serves to partially deactivate ( poison ) the catalyst (so-called Lindlar catalyst ). A catalyst deactivated in this way enables simple hydrogenation of the alkyne to give the underlying alkene and prevents double hydrogenation to give the alkane . Catalysts deactivated with quinoline are also used for the Rosenmund reduction , a palladium-catalyzed reduction of carboxylic acid chlorides to aldehydes.
Hazard warnings
Quinoline is classified as toxic and hazardous to the environment and may only be handled with strong ventilation and only with suitable protective gloves. The release of the substance into the environment must be prevented. There is sufficient evidence that human exposure to quinoline can cause cancer . Furthermore, there is a well-founded suspicion of a mutagenic effect . Quinoline is classified as class 2 hazardous to water .
With air, quinoline forms ignitable air-vapor mixtures from its flash point of 101 ° C. The explosion range is between 1% by volume (54 g / m 3 ) as the lower explosion limit (LEL) and 7% by volume (376 g / m 3 ) as the upper explosion limit (UEL). The ignition temperature is 480 ° C. The substance therefore falls into temperature class T1. In the event of fire, nitrous gases can appear as decomposition products. The electrical conductivity is rather low at 2.2 · 10 −6 S · m −1 .
toxicology
Quinoline has a mean lethal dose of 270 mg · kg −1 after oral ingestion by rats , whereas it is 1400 mg · kg −1 after dermal exposure . In a study on rats, almost all test animals died after seven hours of inhalation of quinoline-saturated air. Acute symptoms of exposure were ataxia and goose bumps . Bleeding and edema in the digestive tract were found in the dead test animals , which can be traced back to quinoline exposure. In studies with rabbits, slight to moderate edema and reddening formed after dermal exposure, but the skin regenerated completely after the end of the test series. Mild to moderate, but also reversible, eye irritation was also observed.
After oral intake, a long-term study in rats and mice showed a carcinogenic effect, which manifested itself in the formation of hepatocellular carcinomas and angiosarcomas . In contrast, these findings did not occur in guinea pigs and hamsters . A slight carcinogenic effect after dermal exposure was found in mice. According to an investigation, the amount of quinoline necessary for the development of carcinomas is 250-300 times higher than that of the strong carcinogen benzapyren . However, quinoline itself is not responsible for the carcinogenic effect, but rather its metabolite . There are known different degradation pathways of quinoline and its derivatives under aerobic and anaerobic conditions. Which path is taken depends on the organism in question. The first degradation step usually consists of an oxidation of the aromatic, which leads, for example, to 2-hydroxyquinoline by aldehyde oxidase or to 3-hydroxyquinoline by cytochrome P450 proteins . Mammals excrete the oxidized breakdown products in a short time via the gastrointestinal tract.
Quinoline in the environment
Traces of quinoline were found in the vicinity of aluminum- smelting plants with associated coking plants and coal-processing plants, which can be attributed to the occurrence of quinoline derivatives in coal. Traces of quinoline are also released during the processing of coal tar and tar oil . Tar oil is or has been used as a rot-inhibiting agent for impregnating wood, for example for railway sleepers or telegraph poles, whereby quinoline can be released into the environment away from the relevant industry. Furthermore, quinoline can be produced as a trace gas in the incomplete combustion of organic nitrogenous compounds.
Quinoline is quickly washed out of contaminated soil by water and broken down by bacteria and humic acids . As a rule, more favorable conditions prevail in water close to the surface, while the degradation in deep water takes place only slowly due to the lack of suitable organisms and unfavorable chemical conditions (among other things due to the low oxygen concentration). Quinoline that is near the surface or in the atmosphere is also subject to decomposition by photolysis . This degradation path is strongly dependent on the photon density, the pH value and the temperature. Depending on the conditions, the half-life of quinoline due to photolytic degradation is between 21 and 160 days. The compound has only a low potential for bioaccumulation because quinoline is rapidly broken down by bacteria, fish and mammals. Model calculations on the distribution of quinoline showed that the compound is essentially transported through water. The transport through the atmosphere takes a back seat due to the low vapor pressure.
proof
The UV / Vis spectrum of quinoline shows three absorption bands . These result from π → π * and n → π * transitions and occur at wavelengths of 226 nm ( extinction coefficient ε = 35,500 l · (mol · cm) −1 ), 270 nm (ε = 3880 l · (mol · cm ) −1 ) and 313 nm (ε = 2360 l (mol cm) −1 ). The infrared spectrum of quinoline shows a large number of absorption bands. Characteristic strong and very strong bands occur at 3333, 1034, 941, 808, 787, 760 and 740 cm −1 . There are also eight other closely spaced, strong absorption bands between 1629 and 1319 cm −1 .
The proton signals in the solvent-free 1 H- NMR spectrum of quinoline lie without exception in a range that is characteristic of aromatic protons. Within this range, however, in relation to benzene, they sometimes show pronounced shifts to lower fields and are an expression of the reduced electron density at these hydrogen atoms. The spectrum shows seven signals corresponding to the seven chemically different protons in the molecule. Since each signal represents a proton, the signals have the same area integrals . The signal at the lowest field results from the proton in the 2-position δ (2-H) = 8.8 ppm, followed by the protons in the 8- (δ (8-H) = 8.1 ppm) and 4-position ( δ (4-H) = 8.0 ppm). The other proton signals are in the 7.7 and 7.3 ppm range. The larger chemical shifts of the protons compared to the carbon analogue naphthalene result from the lower electron density in the aromatic system and correspond relatively to the lower electron densities in these positions, which can be derived from the mesomeric boundary structures. Corresponding to the number of carbon atoms, nine signals appear in the 13 C-NMR spectrum in the range between 122 and 151 ppm. The chemical shifts of the 13 C nuclei behave analogously to the proton signals. The two positions with low electron density, which are located in the vicinity of the nitrogen atom, show the highest downfield shifts (151 and 149 ppm, respectively).
literature
- T. Eicher, S. Hauptmann: The Chemistry of Heterocycles , 2nd edition, Wiley-VCH, Weinheim 2003, ISBN 3-527-30720-6 .
- JA Joule, K. Mills: Heterocyclic Chemistry , 3rd Edition, Blackwell Science, Oxford 2004, ISBN 0-632-05453-0 .
- DT Davies: Basistexte Chemie: Aromatic Heterocyclen , 1st edition, Wiley-VCH, Weinheim 1995, ISBN 3-527-29289-6 .
Web links
Individual evidence
- ↑ a b c Entry on quinoline. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ a b c d O. Eckstein: About quinoline chlorohydrate and the action of acid chlorides on quinoline , in: Chem. Ber. , 1906 , 39 , pp. 2135-2138; doi: 10.1002 / cber.190603902173 .
- ↑ a b c d e f g h David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-454.
- ↑ a b c d e f g h i j Entry on quinoline in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ a b c d e f g h i j k J. A. Joules, K. Mills: Heterocyclic Chemistry , 5th Edition, Blackwell Publishing, Chichester, 2010, ISBN 978-1-4051-9365-8 , pp. 177-199.
- ↑ a b c A. D. Buckingham, JYH Chau, HC Freeman, RJW Le Fèvre, DAAS Narayana Rao, J. Tardif: The dipole moments of pyridine, quinoline, and isoquinoline as vapors and as solutes , in: J. Chem. Soc. , 1956 , pp. 1405-1411; doi: 10.1039 / JR9560001405 .
- ^ A b W. HF Sasse: Synthetical applications of activated metal catalysts. Part VIII. The action of degassed Raney nickel on quinoline and some of its derivatives , in: J. Chem. Soc. , 1960 , pp. 526-533; doi: 10.1039 / JR9600000526 .
- ↑ Entry on quinoline in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b c d data sheet quinoline (PDF) from Merck , accessed on September 5, 2010.
- ^ A b c W. V. Steele, DG Archer, RD Chirico, WB Collier, IA Hossenlopp, A. Nguyen, NK Smith, BE Gammon: The thermodynamic properties of quinoline and isoquinoline , in: J. Chem. Thermodynamics , 1988 , p. 1233 -1264; doi: 10.1016 / 0021-9614 (88) 90161-9 .
- ↑ a b c d e f g h i G. Collin, H. Höke: Quinoline and Isoquinoline , in: Ullmann's Encyclopedia of Industrial Chemistry , Wiley-VCH, Weinheim 2005.
- ↑ a b Ch. Gerhardt : Investigations on the organic bases , in: Annalen der Chemie und Pharmacie , 1942 , 42 , pp. 310-313; doi: 10.1002 / jlac.18420420310 .
- ↑ a b Ch. Gerhardt : Investigations on the chemical classification of organic substances , in: J. Prakt. Chem. , 1843 , 28 , pp. 65-100; doi: 10.1002 / prac.18430280112 .
- ^ Monthly report: Quinoline , in: Archiv der Pharmazie , 1882 , 20 , pp. 50–73; doi: 10.1002 / ardp.18822200109 .
- ↑ a b Paul Rabe: Investigations in the pyridine and quinoline series , in: J. Prakt. Chem. , 1938 , 151 , pp. 65-81; doi: 10.1002 / prac.19381510203 .
- ↑ W. Königs: Oxidation products of cinchonine , in: Chem. Ber. , 1879 , 12 , pp. 79-101; doi: 10.1002 / cber.18790120130 .
- ↑ a b S. Hoogewerff, WA van Dorp: Sur la quinoléine du goudron de houille et des alcaloïdes du quinquina, et sur leur oxydation au moyen du permanganate de potassium , in: Rec. Trav. Chim. , 1882 , 1 , pp. 1-17; doi: 10.1002 / recl.18820010501 .
- ↑ B. Aronheim: Synthesis of Naphthalene , in: Chem. Ber. , 1873 , 6 , pp. 67-68; doi: 10.1002 / cber.18730060125 .
- ↑ a b W. Koenigs: Synthesis of quinoline from allylaniline , in: Chem. Ber. , 1879 , 12 , p. 453; doi: 10.1002 / cber.187901201128 .
- ^ M. Hesse: Alkaloide , pp. 375-378, 1st edition, Verlag Helvetica Chimica Acta, Zurich, 2000, ISBN 3-906390-19-5 .
- ↑ a b c d Toxological Review of Quinoline (PDF; 175 kB) , Environmental Protection Agency , September 2001.
- ↑ a b D. Hellwinkel: The systematic nomenclature of organic chemistry , 4th edition, Springer Verlag, Berlin 1998, ISBN 3-540-63221-2 .
- ^ A. Gossauer: Structure and Reactivity of Biomolecules , 2006 , p. 488, Wiley-VCH Weinheim, ISBN 3-906390-29-2 .
- ↑ G. Lunge : The distillation of the coal tar and the processing of the related by-products , Friedrich Vieweg and Son, Braunschweig, 1867.
- ↑ S. Shimizu, N. Watanabe, T. Kataoka, T. Shoji, N. Abe, S. Morishita, H. Ichimura: Pyridine and Pyridine Derivatives , in: Ullmann's Encyclopedia of Industrial Chemistry , Wiley-VCH, Weinheim 2005.
- ↑ European patent: EP 100109, 1983 , Rütgerswerke .
- ↑ Japanese patent: JP 86161265, 1986 , Sumikin Coke & Chemicals.
- ↑ ZH Skraup: A synthesis of quinoline , in: Chem. Ber. , 1880 , 13 , pp. 2086-2087; doi: 10.1002 / cber.188001302195 .
- ^ A b c D. T. Davies: Basistexte Chemie: Aromatic Heterocyclen , pp. 46–52, 1st edition, Wiley-VCH, Weinheim, 1995, ISBN 3-527-29289-6 .
- ^ O. Doebner, W. v. Miller: About a base homologous to quinoline , in: Chem. Ber. , 1881 , 14 , pp. 2812-2817; doi: 10.1002 / cber.188101402258 .
- ^ A b F. W. Bergstrom, Heterocyclic Nitrogen Compounds. Part IIA. Hexacyclic Compounds: Pyridines, Quinolines, and Isoquinolines. , in: Chem. Rev. , 1944 , 35 , pp. 77-277; doi: 10.1021 / cr60111a001 .
- ^ A. Shaabani, E. Soleimani, Z. Badri: Triflouroacetic Acid as an Efficient Catalyst for the Synthesis of Quinoline , in: Synth. Commun. , 2007 , 37 , pp. 629-635; doi: 10.1080 / 00397910601055230 .
- ↑ C.-S. Jia, Z. Zhang, S.-J. Tu, G.-W. Wang: Rapid and efficient synthesis of poly-substituted quinolines assisted by p-toluene sulphonic acid under solvent-free conditions: comparative study of microwave irradiation versus conventional heating , in: Org. Biomol. Chem. , 2006 , 4 , pp. 104-110; doi: 10.1039 / b513721g .
- ↑ J. Wu, H.-G. Xia, K. Gao: Molecular iodine: a highly efficient catalyst in the synthesis of quinolines via Friedländer annulation , in: Org. Biomol. Chem. , 2006 , 4 , pp. 126-129; doi: 10.1039 / b514635f .
- ↑ R. Varala, R. Enugala, SR Adapa: Efficient and Rapid Friedlander Synthesis of Functionalized Quinolines Catalyzed by Neodymium (III) Nitrate Hexahydrate in: Synthesis , 2006 , pp 3825-3830; doi: 10.1055 / s-2006-950296 .
- ^ RH Manske: The Chemistry of Quinolines. , in: Chem. Rev. , 1942 , 30 , pp. 113-144; doi: 10.1021 / cr60095a006 .
- Jump up ↑ VV Kouznetsov, LYV Méndez, LYV Gómez: Recent Progress in the Synthesis of Quinolines , in: Curr. Org. Chem. , 2005 , 9 , pp. 141-161; doi: 10.1002 / chin.200516245 .
- ↑ RH Reitsema: The Chemistry of 4-Hydroxyquinolines. , in: Chem. Rev. , 1948 , 43 , pp. 43-68; doi: 10.1021 / cr60134a002 .
- ↑ R. Camps: Synthesis of alpha- and gamma-oxyquinolines , in: Chem. Ber. , 1899 , 22 , pp. 3228-3234; doi: 10.1002 / cber.18990320389 .
- ↑ L. Knorr : Synthetic experiments with the acetoacetic ester , in: Liebigs Ann. , 1886 , 236 , pp. 69-115; doi: 10.1002 / jlac.18862360105 .
- ^ RG Gould, WA Jacobs: The Synthesis of Certain Substituted Quinolines and 5,6-Benzoquinolines , in: J. Am. Chem. Soc. , 1939 , 61 , pp. 2890-2895; doi: 10.1021 / ja01265a088 .
- ^ PM Dewick: Medicinal Natural Products: A Biosynthetic Approach , 1st edition, pp. 380-397, John Wiley & Sons, New York, 2009, ISBN 0-470-74167-8 .
- ↑ S. Malanowski: Vapor Pressures and Boiling Temperatures of Some Quinoline Bases in: Bull Acad.. Pole. Sci. Ser. Sci. Chim. , 1961 , 9 , pp. 71-76.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Fluid Properties, pp. 6-675.
- ↑ F. Glaser, H. Ruland: Investigations into vapor pressure curves and critical data of some technically important organic substances , in: Chem. Ing. Techn. 1957 , 29 , p. 772.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Thermochemistry, Electrochemistry, and Solution Chemistry, pp. 5-38.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Fluid Properties, pp. 6-212.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Fluid Properties, pp. 6-223.
- ^ JE Davies, AD Bond: Quinoline , in: Acta Cryst. , 2001 , E57 , pp. 0947-0949; doi: 10.1107 / S1600536801014891 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-448.
- ^ J. Buddrus: Fundamentals of organic chemistry , p. 337, 3rd edition, de Gruyter Verlag, Berlin, 2003, ISBN 3-11-014683-5 .
- ↑ MW Austin, JH Ridd: The kinetics and mechanism of heteroaromatic nitration. Part I. Quinoline , in: J. Chem. Soc. , 1963 , pp. 4204-4210; doi: 10.1039 / JR9630004204 .
- ↑ GE McCasland: The Preparation of 8-Quinolinesulfonic acid , in: J. Org. Chem. , 1946 , 11 , pp. 277-280; doi: 10.1021 / jo01173a010 .
- ↑ JL Butler, M. Gordon: A reinvestigation of known bromination reactions of quinoline , in: J. Heterocycl. Chem. , 1975 , 12 , pp. 1015-1020; doi: 10.1002 / jhet.5570120539 .
- ^ TJ Kress, SM Constantino: Selective brominations in nitrobenzene. A convenient synthesis of 3-brornoquinoline, 4-bromoisoquinoline, and 4-phenyl-5-bromopyrimidine , in: J. Heterocycl. Chem. , 1973 , 10 , pp. 409-410; doi: 10.1002 / jhet.5570100326 .
- ↑ H. van der Plas: Oxidative Amino-Dehydrogenation of Azines , in: Adv. Heterocycl. Chem. , 2004 , 86 , pp. 1-40; doi: 10.1016 / S0065-2725 (03) 86001-4 .
- ↑ JA Zoltewicz, LS Helmick, TM Oestreich, RW King, PE Kandetzki: Addition of amide ion to isoquinoline and quinoline in liquid ammonia. Nuclear magnetic resonance spectra of anionic σ-complexes , in: J. Org. Chem. , 1973 , 38 , pp. 1947-1949; doi: 10.1021 / jo00950a036 .
- ^ TJ Geissmann, MJ Schlatter, ID Webb, JD Roberts: The Synthesis of some Intermediates for the Use in the Preparation of Analogs of Salicylaldehyde ethylenediimine cobalt ("Salcomine") , in: J. Org. Chem. , 1946 , 11 , p 741-750; doi: 10.1021 / jo01176a015 .
- ↑ M. Ishikura, T. Mano, I. Oda, M. Terashima: An Alternative Synthesis of Dialkylpyridylboranes , in: Heterocycles , 1984 , pp. 4271-4274; doi: 10.3987 / R-1984-11-2471 .
- ↑ H. Gilman , T. Soddy: Notes - Some Organolithium Compounds of Quinoline and 2-Phenylquinoline , in: J. Org. Chem. , 1958 , 23 , pp. 1584-1585; doi: 10.1021 / jo01104a627 .
- ↑ JB Wommack, TG Barbee Jr., DJ Thoennes, MA McDonald, DE Pearson: The synthesis of quinoline- and isoquinolinecarboxaldehydes , in: J. Heterocycl. Chem , 1969 , 6 , pp. 243-245; doi: 10.1002 / jhet.5570060217 .
- ↑ J. Meisenheimer : About pyridine, quinoline and isoquinoline-N-oxide. , in: Ber. German Chem. Ges. , 1926 , 59 , pp. 1848-1853; doi: 10.1002 / cber.19260590828 .
- ↑ CF Koelsch, AF Steinhauer: Synthesis, Nitration, and Oxidation of 3-Azafluoranthene , in: J. Org. Chem. , 1953 , 18 , pp. 1516-1522; doi: 10.1021 / jo50017a010 .
- ↑ a b c J. C. Cochran, WF Little: Electrolytic Oxidation of Some Substituted Quinolines to Quinolinic Acids and Acylations with Substituted Quinolinic Anhydrides , in: J. Org. Chem. , 1961 , 21 , pp. 808-811; doi: 10.1021 / jo01062a039 .
- ↑ G. Queguiner, P. Pastour: Synthesis dans la série de la pyridine - I - Les diformylpyridines , in: Bull. Soc. Chim. Fr. , 1968 , 10 , pp. 4117-4121; OCLC 491832299 .
- ↑ C. O'Murchu: Ozonolysis of Quinolines: A Versatile Synthesis of Polyfunctional Pyridines , in: Synthesis , 1989 , 11 , pp. 880-882; doi: 10.1055 / s-1989-27423 .
- ↑ GR Girard, WE Bondinell, LM Hillegass, KG Holden, RG Pendleton, I. Uzinskas: Tetrahydro thiadiazolo isoquinolines: synthesis and inhibition of phenylethanolamine-N-methyltransferase , in: J. Med. Chem. , 1989 , 32 , p. 1566 -1571; doi: 10.1021 / jm00127a027 .
- ↑ A. Nose, T. Kudo: Reduction of Heterocyclic Compounds. II. Reduction of Heterocyclic Compounds with Sodium Borohydride-Transition Metal Salt Systems , in: Chem. Pharm. Bull. , 1984 , 32 , pp. 2421-2425; Abstract .
- ^ BC Ranua, U. Jana, A. Sarkara: Regioselective Reduction of Quinolines and Related Systems to 1,2,3,4-Tetrahydro Derivatives with Zinc Borohydride , in: Synth. Commun. , 1998 , 28 , pp. 485-492; doi: 10.1080 / 00397919808005103 .
- ↑ GL Patrick: Synthesis of (±) - [4a α , 4b β , 10b β , 12a α ] -9-halogeno-2-methyl-1,2,3,4,4a, 4b, 5,6,10b, 11,12,12a-dodecahydronaphtho [2,1- f ] isoquinolines , in: J. Chem. Soc., Perkin Trans. 1 , 1995 , pp. 1273-1279; doi: 10.1039 / P19950001273 .
- ^ EA Braude, J. Hannah, Sir R. Linstead: Hydrogen transfer. Part XVI. Dihydrides of nitrogenous heterocycles as hydrogen donors , in: J. Chem. Soc. , 1960 , pp. 3249-3257; doi: 10.1039 / JR9600003249 .
- ↑ Arthur Birch , PG Lehmann: 1,4-Dihydroquinoline , in: Tetrahedron Lett. , 1974 , 15 , pp. 2395-2396; doi: 10.1016 / S0040-4039 (01) 92265-8 .
- ↑ MF Depompei, A. Hlynsky, Diamond Shamrock, US 4,281,125., 1980
- ↑ JP Senet, G. Sennvey, G. Wooden, Société Nationale des Poudres et Explosifs, EP 249 556, 1987.
- ↑ NN Woroshtzow, JM Kogan: On the effect of sulphurous acid and its salts on quinoline derivatives , in: Ber. German Chem. Ges. , 1930 , pp. 2354-2362; doi: 10.1002 / cber.19300630878 .
- ↑ PL Orwick, AR Templeton, American Cyanamid, EP 41 623, 41 624, 1981.
- ^ A. Streitwieser, CH Heathcock, EM Kosower: Organische Chemie , 2nd edition, Wiley-VCH, Weinheim 1994, ISBN 3-527-29005-2 , p. 1227.
- ↑ S. Hoogewerff, WA van Dorp: About the oxidation of quinoline by means of potassium permanganate , in: Chem. Ber. , 1879 , 12 , pp. 747-748; doi: 10.1002 / cber.187901201208 .
- ↑ W. Orth, E. Pastorek, W. Fickert, Rütgerswerke, EP 149857., 1984
- ↑ RWJ Rebhahn, JE Kassner, RE Werner, Hilton-Davis Chemical, US 4,537,971., 1985
- ↑ S. Murahashi, Sumitomo Chemical, JP 87 212 363, 1986.
- ↑ R. Lattrell, W. Dürckheimer, R. Kirstetter, Hoechst, DE 3 706020, 1987.
- ^ H. Lindlar, R. Dubuis: Palladium Catalyst for Partial Reduction of Acetylenes In: Organic Syntheses . 46, 1966, p. 89, doi : 10.15227 / orgsyn.046.0089 ; Coll. Vol. 5, 1973, p. 880 ( PDF ).
- ↑ H. Lindlar: A new catalyst for selective hydrogenation , in: Helv. Chim. Acta , 1952 , 35 , pp. 446-450; doi: 10.1002 / hlca.19520350205 .
- ^ J. Buddrus: Fundamentals of Organic Chemistry , 3rd edition, de Gruyter Verlag, Berlin 2003, ISBN 3-11-014683-5 , p. 295.
- ^ J. Buddrus: Fundamentals of Organic Chemistry , 3rd edition, de Gruyter Verlag, Berlin 2003, ISBN 3-11-014683-5 , p. 538.
- ^ E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ Technical rule for operational safety - TRBS 2153, BG RCI leaflet T033 Avoidance of ignition hazards due to electrostatic charges , as of April 2009, Jedermann-Verlag Heidelberg.
- ↑ K. Hirao, Y. Shinohara, H. Tsuda, S. Fukushima, M. Takahashi, N. Ito: Carcinogenic Activity of Quinoline on Rat Liver , in: Cancer Res. , 1976 , 36 , pp. 329-335; PMID 177193 ( full text access ; PDF; 1.3 MB).
- ↑ a b Y. Shinohara, T. Ogiso, M. Hananouchi, K. Nakanishi, T. Yoshimura, N. Ito: Effect of various Factors on the Induction of Liver Tumors in Animals by Quinoline , in: GANN , 1977 , 68 , Pp. 785-796; PMID 598648 .
- ^ EJ LaVoie, A. Shigematsu, EA Adams, J. Rigotty, D. Hoffmann: Tumor-initiating activity of quinoline and methylated quinolines on the skin of SENCAR mice , in: Cancer Lett. , 1984 , 22 , pp. 269-273; doi: 10.1016 / 0304-3835 (84) 90162-9 ; PMID 6324986 .
- ↑ MM Ramirez-Corredores, AP Borole: Studies in Surface Science and Catalysis 164: Biocatalysis in Oil Refining , 1st edition, pp. 155-160, Elsevier Verlag, New York, 2007, ISBN 0-444-52212-3 .
- ↑ P. Jenner, B. Testa: Concepts in Drug Metabolism: Part A , 3rd Edition, p. 115, Marcel Dekker, New York, 1980, ISBN 0-8247-6906-6 .
- ↑ L. Novack, BB Brodie: Quinoline and its transformation products found in urine , in: J. Biol. Chem. , 1950 , 187 , pp. 787-792 ( full text access ).
- ^ JT Smith, RN Williams: Studies in detoxication. 65. The metabolism of quinoline. New metabolites of quinoline, with observations on the metabolism of 3-, 5- and 6-hydroxyquinoline and 2: 4-dihydroxyquinoline , in: Biochem. J. , 1955 , 60 , pp. 284-290; PMC 1215695 (free full text, PDF).
- ↑ a b Investigation report of the state of Canada
- ↑ a b J. A. Joule, K. Mills: Heterocyclic Chemistry , pp. 14-16, 3rd edition, 2004 , Blackwell Science, Oxford, ISBN 0-632-05453-0 .
- ↑ H. Tschammler, H. Krischai: Quinoline- m- cresol, a strongly negative system. , in: monthly Chem. , 1951 , 82 , pp. 259-270; doi: 10.1007 / BF00899511 .