ipso substitution
The term ipso substitution is often used in organic chemistry in connection with a special mechanism of electrophilic aromatic substitution . The term “ ipso ” (from Latin for “self”) is intended to express that the substituent X itself is replaced by the electrophile . According to the IUPAC recommendation, however, the expression " ipso -substitution" should be avoided, since it is a pleonasm . The correct designation of an aromatic substitution mechanism includes electrostatic information, molecularity and regioselectivity , S E Ar 2 ipso thus defines the mechanism dealt with here as bimolecular electrophilic aromatic substitution under ipso attack. Ipso attacks can, however, also appear in other substitution reactions. cine and tele attack are related names for other substitution patterns.
Substituents
In the case of aromatic substitutions, existing substituents play an important role in the reactivity of the overall compound. They have a decisive influence on a second substitution and determine at which point the ring system is preferably further substituted. If instead of a substitution in the vicinity of an already existing substituent, the attacking electrophile attacks the first substituent itself, one speaks of ipso substitution. This only occurs if X is a better leaving group in the ring system than a proton , which itself is an excellent leaving group.
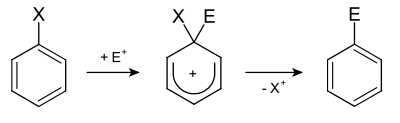
General mechanism
The general mechanism of an S E Ar 2 ipso reaction can be formulated as follows:
The probability of an ipso attack is greater, the more the substituent directs the reaction into a position in which it can function as a leaving group itself. Silyl groups are predestined for this, as they stabilize the negative charge in the alpha position.
An example of an ipso reaction is the reversibility of the sulfonation .
Proof of the ipso intermediate level
In some cases the cationic intermediate could be isolated by trapping reactions. An example of this is the nitration of o -xylene with nitric acid in acetic anhydride (MeCO) 2 O. In addition to the nitryl ion, the acetate anion MeCO 2 - is formed , which attacks the intermediate stage and converts it into a stable addition product.
In terms of preparation, ipso substitution plays a rather subordinate role compared to the important ortho , meta and para substitution, but it is to be expected especially when an electrophilic substitution is carried out on an already substituted aromatic .
Individual evidence
- ↑ Entry on ipso-attack . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.I03251 Version: 2.1.5.
literature
- Peter Sykes: How do organic reactions work? 2nd edition, Wiley-VCH 2001, ISBN 3-527-30305-7 .
- H.-PE Kohler, FLP Gabriel, W. Giger : ipso-Substitution - A Novel Pathway for Microbial Metabolism of Endocrine-Disrupting 4-Nonylphenols, 4-Alkoxyphenols, and Bisphenol A. Chimia 62 (2008), 358-363. doi : 10.2533 / chimia.2008.358 .