Silanes
According to the IUPAC rules, the term silane stands for a group of chemical compounds that consist of a silicon skeleton and hydrogen . Similar groups of substances are Germans and Alkanes . Silanes can have a branched ( iso - and neo -silanes ) or unbranched ( n -silanes ) structure have. The general empirical formula of the acyclic (open-chain, also called catena-silanes) silanes is Si n H 2n + 2 . Ring-shaped silicon-hydrogen compounds are called cyclosilanes ( general empirical formula : Si n H 2n ).
history
In 1857 Friedrich Wöhler and Heinrich Buff produced a gaseous silicon hydrogen compound (presumably monosilane) for the first time during the anodic decomposition of a silicon-rich aluminum rod in various salt solutions ( sodium , ammonium or potassium chloride ) by means of an electric current, which contained the hydrogen as an impurity and this when it came into contact with air inflamed. On the one hand, the gas mixture could be stored unchanged over a salt solution, but exploded immediately upon contact with atmospheric oxygen with a glowing flame, forming a white solid, which Wöhler identified as silicon dioxide . The reactive gas from the mixture deposited solid silicon on a heated platinum wire with elimination of hydrogen. The attempt to reproduce this new silicon hydrogen compound by reacting crystalline silicon beneath the red heat with hydrogen chloride gas did not succeed Wöhler, instead he synthesized the first chlorosilanes. In the following year, Wöhler then optimized the synthesis of silicon hydrides by reacting magnesium silicide (Mg 2 Si) with concentrated hydrochloric acid .
In 1902 the idea of silicon hydrides was taken up again. Henri Moissan succeeded in detecting monosilane and disilane after the protolysis of lithium silicide . From 1916 onwards, Alfred Stock , at that time at the Kaiser Wilhelm Institute for Chemistry in Berlin-Dahlem, dealt intensively with silicon hydride chemistry and within 9 years published, largely together with Carl Somieski , a series of publications on silicon hydride with 16 issues. By reacting magnesium silicide with acid, analogous to the synthesis by Wöhler, Stock and Somieski first achieved the preparation of the lower gaseous silanes monosilane and disilane as well as the liquids trisilane and tetrasilane . Since then these silanes have been referred to as "Stock's silanes". Stock gave the entire group of substances the general name Silane . He also proved the existence of pentasilane and hexasilane and produced various halosilanes and siloxanes from them.
Alfred Stock designed a glass apparatus to represent the silanes, which allowed work to be carried out under complete exclusion of air. He used a glass flask that was half filled with aqueous sulfuric acid , stirred the acid and poured in the ground, gray magnesium silicide in portions. The silicide decomposed with the development of heat and the formation of gaseous hydrogen and silanes. Stock now conducted the gases into a glass apparatus, which he cooled from the outside. The cooling was set so that the hydrogen and the monosilane could not condense. With the cooling, he hoped that any longer-chain silanes would condense due to their higher boiling points . In fact, he succeeded in obtaining a water-clear liquid that was composed of three chain-shaped silanes. However, further derivatization of these compounds was unsuccessful.
Extraction and presentation
There are essentially three main ways in which silanes are made. In the form of the so-called raw silane through the decomposition of magnesium silicide (Mg 2 Si) under acidic conditions and with the exclusion of air, analogous to the syntheses by Wöhler and Stock.
By reducing the corresponding halosilanes with lithium hydride or lithium aluminum hydride in an organic solvent such as diethyl ether . In this way the corresponding silanes Si n H 2n + 2 with n = 1,2,3 can be produced selectively and in high yield.
The third way is the reaction of elemental silicon with hydrogen halides or alkyl halides in the presence of copper as a catalyst, similar to Wöhler's process, in which halosilanes or alkylhalosilanes are formed.
In addition, trisilane and higher silanes can be obtained in good yield from monosilane with the aid of an electrical discharge. Depending on the synthesis conditions, polysiline (SiH 2 ) ∞ , the silicon homologue of the alkenes , or polysiline (SiH) ∞ (compare alkynes ) are formed. Compounds with an intermediate stoichiometry [(SiH n ) ∞ , 1 n 2] and ring-shaped oligo- and polysilanes are also possible.
nomenclature
The name is given with the root word Sil and the suffix -an , analogous to the saturated aliphatic hydrocarbons that also end in -an (methane, ethane, propane, butane and so on). The number of silicon atoms is placed in front as a Greek numerical word: monosilane (one silicon atom), disilane (two silicon atoms), trisilane, etc. If a silane contains four or more silicon atoms, different arrangements, more precisely constitutions , are possible. One speaks of Constitution isomerie . For further differentiation, depending on the structure of the molecule , one of the descriptors n- , iso- , neo- or cyclo- demic compound names is placed in front.
Structure and properties
Silanes are the silicon homologues of alkanes based on a carbon framework . They have similar physical properties, such as melting point, boiling point and dipole moment, as the corresponding alkanes. Like saturated hydrocarbons , silanes generally have a tetradhedral geometry based on sp 3 hybridization of the bond orbitals. However, there are fundamental differences between silicon hydride and hydrocarbon chemistry. Since silicon is more electropositive than carbon and hydrogen, the bonds in silanes are usually more polarized than in the analogous hydrocarbon compounds. The bonded hydrogen atoms have an electronegative character and therefore react easily with acidic hydrogen. The Si-H bond energy of 378 k J / mol is significantly lower than the CH bond energy of 414 kJ / mol, which means that silanes are (thermally) less stable than alkanes.
There are far fewer silanes than there are hydrocarbons. So far only unbranched and branched silanes with up to 8 and cyclic silanes with 5 or 6 silicon atoms are known. All of them are colorless gases or liquids. Compared to the parent compounds, polynuclear silanes become much more stable through substitution with halogens or organic residues.
Homologous series of the linear silanes
The homologous series of linear, unbranched silanes results from the general formula H− (SiH 2 ) n −H with n = 1, 2, 3, ... The lowest silanes - monosilane and disilane - are gaseous. From trisilane on, the silanes assume a liquid state of aggregation .
| Silane | Molecular formula |
Melting point in ° C |
Boiling point in ° C |
Density in g / cm³ |
Molar mass in g / mol |
|---|---|---|---|---|---|
| Monosilane | SiH 4 | −184.7 | −112.1 | 0.00135 | 32.12 |
| Disilane | Si 2 H 6 | −129.4 | −14.8 | 0.00287 | 62.22 |
| Trisilane | Si 3 H 8 | −116.9 | 52.9 | 0.739 | 92.32 |
| Tetrasilane | Si 4 H 10 | −91.6 | 108.4 | 0.795 | 122.42 |
| Pentasilane | Si 5 H 12 | −72.2 | 153.2 | 0.827 | 152.52 |
| Hexasilane | Si 6 H 14 | −44.7 | 193.6 | 0.847 | 182.62 |
| Heptasilane | Si 7 H 16 | −30.1 | 226.8 | 0.859 | 212.73 |
| Octasilane | Si 8 H 18 | 242.83 |
Only the monosilane can be stored indefinitely at room temperature and is the most stable of the silanes. In the absence of catalysts, it only decomposes at 500 ° C. The higher homologues are less and less thermally stable and decompose even at room temperature in daylight, releasing hydrogen and forming the shorter homologues and other polymer products ((SiH <2 ) x ). Higher temperatures lead to the breakdown into the elements. Monosilane is correspondingly also formed during the thermolysis of higher silanes and substituted silanes such as trichlorosilane or triethoxysilane .
Isomeric and cyclic silanes
Similar to the alkanes , the occurrence of constitutional isomers is observed with the higher silanes (from tetrasilane) . Branched and cyclic silanes are known. The constitutional isomers differ in various physical characteristics such as melting and boiling points .
| Silane | Molecular formula | Structural formula |
Melting point in ° C |
Boiling point in ° C |
density |
Molar mass in g / mol |
|
|---|---|---|---|---|---|---|---|
| Branched connections | |||||||
| iso- tetrasilane | Si 4 H 10 | 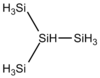 |
−99.4 | +101.7 | 0.793 | 122.42 | |
| iso -pentasilane | Si 5 H 12 | −109.8 | +146.2 | 0.820 | 152.52 | ||
| neo -pentasilane | Si 5 H 12 | −57.8 | +130 | - | 152.52 | ||
| Cyclic silanes | |||||||
| Cyclopentasilane | Si 5 H 10 | 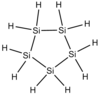 |
−10.5 | +194.3 | 0.963 | 150.51 | |
| Cyclohexasilane | Si 6 H 12 |  |
+16.5 | +226 | - | 180.61 | |
Chemical reaction
In contrast to the homologous alkanes, silanes are less stable. They can only be synthesized in the absence of air . The lower silanes Si n H 2n + 2 (with n = 1 to 4) are very reactive and can self-ignite or explode in air, burning to silicon dioxide and water .
The reactivity decreases with increasing chain length. Even pentasilane no longer reacts independently with the oxygen content of the air. As of heptasilane , silanes are no longer spontaneously self-igniting.
Silanes, on the other hand, are largely stable to (oxygen-free) water and can therefore be stored for longer periods in salt solutions. In water with a pH value above 7, silanes slowly decompose to form silica and hydrogen, while strongly basic solutions split off spontaneously and quantitatively hydrogen. The reaction can be controlled in such a way that one molecule of hydrogen is released for each Si – H and Si – Si bond, whereby the molar composition can be determined.
The hydrolysis of organosilanes is a first-order reaction . The rate of hydrolysis of the Si-H bond depends on the type and number of organic residues. The hydrolysis of trialkylsilanes is significantly slower than that of triarylsilanes. This can be explained by a greater increase in the electron density on the silicon atom due to the alkyl groups. The reaction rate of the tri-n-alkylsilanes decreases accordingly in the series ethyl, propyl, butyl. In the case of trialkylsilanes, the silanes with n-alkyl radicals react 10 times faster than the analogous silanes with branched alkyl radicals.
Monosilane reacts with methanol to form di-, tri- and tetramethoxymonosilane but not to the corresponding monomethoxy compound.
The corresponding alkali silyl derivatives are formed with alkali metals dissolved in solvents . Depending on the solvent, two competing reactions can take place. While both processes take place in 1,2-dimethoxyethane , only the second reaction takes place in hexamethylphosphoramide . In contrast, ammonolysis takes place in liquid ammonia as a solvent .
With organolithium compounds , silanes form the corresponding alkylsilanes, the peralkylated silanes being preferentially formed.
Silanes can also react with nitrogen by decomposing at temperatures of around 500 ° C and the resulting silicon reacts with nitrogen. Also, microwave radiation can be used for decomposition. This reaction is used, for example, to produce thin silicon nitride layers or silicon nitride nanocrystals.
Derivatives of silanes
Derivatives (descendants) of the silanes are formally formed by exchanging ( substituting ) the hydrogen atoms for other elements or groups. In particular, halogens , oxygen , nitrogen and carbon should be mentioned here.
Halosilanes
Well-known representatives are the chlorosilanes monochlorosilane (SiH 3 Cl), dichlorosilane (SiH 2 Cl 2 ), trichlorosilane (SiHCl 3 ) and tetrachlorosilane (SiCl 4 ). Trichlorosilane, in particular, is important on an industrial scale because it is easy to manufacture and is used as a starting material for the manufacture of monosilane, dichlorosilane and high-purity silicon metal. In addition to the production from silicon and hydrogen halide according to Wöhler's instructions, they can also be prepared by reacting silanes with anhydrous hydrogen halide in the presence of aluminum chloride at moderate temperatures.
While the reaction of monosilane with chlorine or bromine is explosive at room temperature, it can be carried out in a controlled manner at lower temperatures.
Organosilanes
If the hydrogen in the silanes is replaced by organic residues , one obtains organosilicon compounds which, according to IUPAC, are regarded as silane derivatives . The silicon – carbon bond is very stable and as early as 1917 Stock reported the existence of more than 50 known tetraalkyl and tetraarylsilanes. These are thermally stable, resistant to water and chemically so stable that they can be halogenated, nitrided or sulfonated on the organic residue without the Si – C bond being split. An important representative is the largely chemically inert tetramethylsilane , which is used as a standard in nuclear magnetic resonance spectroscopy . Due to the stability of the Si – C bond, substances such as hexaphenyldisilane can also be produced whose carbon analogues such as hexaphenylethane do not exist. Organosilanes can be produced by reacting halosilanes or silanes with organolithium compounds or via a Grignard reaction.
Organohalosilanes are produced on an industrial scale using the Müller-Rochow synthesis .
Silanols, siloxanes
By hydrolysis - especially of halosilanes - the silanols are easily obtained , which, in contrast to the analogous alcohols , are not very stable. They polymerize with elimination of water to form siloxanes and polysiloxanes , with hydrogen chloride accelerating the polymerization. Monosilanols form dimers, silanediols polymeric chains and silanediols form three-dimensional networks. The driving force is the formation of particularly stable Si – O – Si bonds.
use
Trichlorosilane is an intermediate product for the production of high-purity silicon for integrated circuits (microchips) , for adhesion promoters and for surface finishing. The thermolysis of silanes in a hydrogen atmosphere also produces high-purity silicon layers ( Siemens process ).
From chlorosilanes and chloroalkylsilanes, so-called fumed silica can be produced by reaction in an oxyhydrogen flame , which is an important filler for plastics , among other things . Special silanes called functional organosilanes are used for surface functionalization, which is also known as silanization for short . Silanes from the range of chloromethylsilanes , especially dichlorodimethylsilane , are used as starting materials for the production of silicones .
By oxidizing dichlorosilane under reduced pressure, silicon dioxide can be deposited in thin layers.
Higher, no longer self-igniting silanes have been discussed for their use as rocket fuel . The high price and the lower specific impulse compared to hydrazine or oxyhydrogen mixtures speak against silanes. However, higher, liquid silanes such as pentasilane could be useful as non-toxic additives to increase the combustion efficiency of conventional rocket fuels.
In semiconductor technology , with a suitable PECVD process, dielectric layers made of silicon dioxide from monosilane and z. B. nitric oxide can be produced.
See also
- Silazanes
- Siloxanes
- Alexium International , manufacturer
Web links
- Fire and Flame , Fire and Flame, experiment with Silane (Youtube video with Peter Wothers , English, 2 min.)
Individual evidence
- ↑ F. Wöhler, H. Buff: About a connection of silicon with hydrogen . In: Annals of Chemistry and Pharmacy . tape 103 , no. 2 , 1857, pp. 218-229 , doi : 10.1002 / jlac.18571030213 .
- ↑ a b H. Buff, F. Wöhler: About new compounds of silicon . In: Annals of Chemistry and Pharmacy . tape 104 , no. 1 , 1857, pp. 94-109 , doi : 10.1002 / jlac.18571040108 .
- ↑ F. Wöhler: About the silicon hydrogen gas . In: Annals of Chemistry and Pharmacy . tape 107 , no. 1 , 1858, p. 112 , doi : 10.1002 / jlac.18581070117 .
- ^ A b Egon Wiberg: Alfred Stock 1876-1946 . In: Chemical Reports . tape 83 , no. 6 , October 1950, p. XIX – LXXVI , doi : 10.1002 / cber.19500830619 .
- ^ A b Alfred Stock, Carl Somieski: Siliciumwasserstoffe. I. The silicon hydrides formed from magnesium silicide and acids . In: Reports of the German Chemical Society . tape 49 , no. 1 , January 1916, p. 111-157 , doi : 10.1002 / cber.19160490114 .
- ↑ Alfred Stock: The nomenclature of silicon and boron compounds . In: Reports of the German Chemical Society . tape 49 , no. 1 , January 1916, p. 108-111 , doi : 10.1002 / cber.19160490113 .
- ^ A b c d NN Greenwood, A. Earnshaw: Chemistry of the Elements . Elsevier, 2012, ISBN 978-0-08-050109-3 , pp. 337– ( limited preview in Google Book search).
- ↑ Patent DE3506071 : METHOD FOR PRODUCING DISILANE BY THE REDUCTION OF HEXACHLOROUS DISILANE. Applied on February 21, 1985 , published on August 22, 1985 , applicant: CENTRAL GLASS CO LTD, inventor: AONO KOJI, SAITO TOSHINORI, OKADA CHIHARU.
- ↑ a b c d e f g h i j k l m n o p q Barry Arkles: Silanes. (PDF) Reprint from Kirk-Othmer Encyclopedia of Chemical Technology, Forth Edition, Volume 22, Page 38-69. In: Gelest. P. 39 , accessed on December 10, 2016 (English).
- ↑ a b c d e f Alfred Stock: On the nomenclature of silicon compounds . In: Reports of the German Chemical Society . tape 50 , no. 1 , January 1917, p. 170-182 , doi : 10.1002 / cber.19170500127 .
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 485.
- ↑ a b Bernhard Hidding: Investigation of the suitability of silanes as fuels in the aerospace industry. ( Memento from March 4, 2016 in the Internet Archive ) (PDF; 4.5 MB) Diploma thesis at the University of the Federal Armed Forces in Munich and the Heinrich Heine University in Düsseldorf , January 2004.
- ↑ Alfred Stock, Paul Stiebeler, Friedrich Zeidler: Siliciumwasserstoffe, XVI .: The higher silicon hydrides . In: Reports of the German Chemical Society . tape 56 , no. 7 , July 4, 1923, p. 1695-1705 , doi : 10.1002 / cber.19230560735 .
- ↑ Patent DE10059625 : Process for the production of higher silanes with regard to their use as fuels. Registered on December 1, 2000 , published on May 16, 2002 , applicant: Peter Plichta, inventor: Peter Plichta .
- ↑ G. Schott, C. Harzdorf: Silanes. I Alkaline solvolysis of triorganosilanes . In: Journal of Inorganic and General Chemistry . tape 306 , no. 3-4 , October 1960, pp. 180-190 , doi : 10.1002 / zaac.19603060306 .
- ↑ Jingwei Song, Xiying Ma, Wang Zui, Chen Wei, Zhongpin Chen: Fabrication of Si 3 N 4 Nanocrystals and Nanowires Using PECVD. In: Advances in Materials Science and Engineering. 2010, doi: 10.1155 / 2010/892792 .
- ↑ JR Flemish, RL Pfeffer: Low hydrogen content silicon nitride films from electron cyclotron resonance plasmas. In: J. Appl. Phys. 1993, 74, pp. 3277-3282, doi: 10.1063 / 1.355318 .
- ↑ Joseph Goubeau , Rudolf Warncke: For the hydrolysis of halides. I. The hydrolysis of silicon tetrachloride . In: Journal of Inorganic and General Chemistry . tape 259 , no. 1-4 , October 1949, pp. 109-120 , doi : 10.1002 / zaac.19492590109 .
- ↑ B. Leitenberger: Chemical rocket propellants, part 1.
- ↑ Eugen Unger: The production of thin layers. The PECVD process: vapor deposition in a plasma . In: Chemistry in Our Time . tape 25 , no. 3 , June 1, 1991, pp. 148–158 , doi : 10.1002 / ciuz.19910250306 .









![{\ displaystyle \ mathrm {SiH_ {4} {\ xrightarrow [{- H_ {2}}] {CH_ {3} OH}} \ H_ {2} Si (OCH_ {3}) _ {2}; \ \ HSi (OCH_ {3}) _ {3}; \ \ Si (OCH_ {3}) _ {4}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d0f6d5f6b651ce9b9e0e573afe4b0373e57106ce)






![{\ displaystyle \ mathrm {2 \ ClSi (CH_ {3}) _ {3} {\ xrightarrow [{- HCl}] {+ H_ {2} O}} \ 2 \ HOSi (CH_ {3}) _ {3 } \ {\ xrightarrow [{- H_ {2} O}] {\}} \ (CH_ {3}) _ {3} Si {-} O {-} Si (CH_ {3}) _ {3}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/fd8e40a73751c1524ac00460bd1e26d8de5018e5)