Pentasilane
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
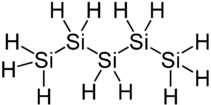
|
||||||||||
| General | ||||||||||
| Surname | Pentasilane | |||||||||
| other names |
n -pentasilane |
|||||||||
| Molecular formula | Si 5 H 12 | |||||||||
| Brief description |
colorless, self-igniting liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 152.52 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
0.827 g cm −3 |
|||||||||
| Melting point |
−72.2 ° C |
|||||||||
| boiling point |
153.2 ° C |
|||||||||
| solubility |
Decomposes in water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Pentasilane is a chemical compound of the elements silicon and hydrogen and belongs to the group of silanes . The substance with the semi- structural formula SiH 3 - (SiH 2 ) 3 –SiH 3 is a colorless liquid that decomposes at room temperature, which on contact with water reacts to form silicic acids and hydrogen, with atmospheric oxygen to form silicon dioxide and water with spontaneous combustion. Pentasilane is the silicon analogue of n -pentane (C 5 H 12 ).
Presentation and reactions
Pentasilane can be synthesized from hexachlorodisilane (Cl 3 Si – SiCl 3 ) and a Lewis base . As a Lewis base z. B. trimethylamine or organophosphorus compounds in question. In the strongly Lewis acidic hexachlorodisilane, the Si-Si bond is cleaved, releasing silicon tetrachloride (SiCl 4 ) and a base-stabilized silylene (X 2 Si-R). The silylene intermediate could also be detected, so that the reaction mechanism was confirmed.
When pentasilane dissolved in 2,3-dimethylbutane is irradiated with UV light , higher homologues such as 3-silylhexasilane and 4-silylheptasilane are formed .
Recent studies suggest the use of higher, liquid silanes such as pentasilane as a non-toxic additive to rocket fuels , as this can increase combustion efficiency.
Individual evidence
- ↑ a b c David R. Lide: CRC Handbook of Chemistry and Physics . 87th edition, CRC Press, 1998, ISBN 9780849305948 , pp. 4-87.
- ↑ a b c Bernhard Hidding: Investigation of the suitability of silanes as fuels in the aerospace industry. (PDF; 4.5 MB) ( Memento of the original from March 4, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Diploma thesis at the University of the Federal Armed Forces Munich and the Heinrich Heine University Düsseldorf , January 2004
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 485.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 940-941.
- ↑ Meyer-Wegner, F .; Nadj, A .; Bolte, M .; Auner, N .; Wagner, M .; Holthausen, MC; Lerner, H.-W .: The Perchlorinated Silanes Si 2 Cl 6 and Si 3 Cl 8 as Sources of SiCl 2 In: Chemistry- A European Journal No. 17, 2011, pp. 4715-4719, doi : 10.1002 / chem. 201003654 .
- ↑ F. Fehéar, I. Fischer: to the chemistry of silicon and germanium. XXX [1]. The photochemical disproportionation of iso- and n-pentasilane. Representation of some new branched hepta- and octasilanes. In: ZAAC 1980 , 466 , 23-28, doi : 10.1002 / zaac.19804660103 .