Magnesium silicide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
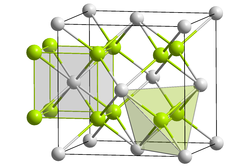
|
||||||||||||||||
| __ Mg 2+ __ Si 4− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Magnesium silicide | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | Mg 2 Si | |||||||||||||||
| Brief description |
slate blue solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 76.70 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.94 g cm −3 |
|||||||||||||||
| Melting point |
1085 ° C |
|||||||||||||||
| solubility |
reacts violently with water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Magnesium silicide is an inorganic chemical compound of magnesium from the group of silicides .
Extraction and presentation
Magnesium silicide can be obtained by reacting magnesium with silicon.
It is also possible to display it by reacting silicon dioxide with magnesium.
Pure phase magnesium silicide can be produced from magnesium hydride and silicon with the help of the SPS process .
properties
Magnesium silicide is a solid with hard and very brittle, slate-blue crystals. It represents a Zintl phase and was first mentioned in 1858 by Friedrich Wöhler . The compound was first investigated in more detail by Paul Lebeau and Robert Bossuet in 1908, who succeeded in producing the purest synthesis of magnesium silicide. It reacts violently with water, producing extremely flammable gases. Above 723 K, the compound reacts with oxygen in air to form magnesium oxide and silicon . It has a crystal structure of the antifluorite type (a = 6.39 Å) with the space group Fm 3 m (space group no. 225) and is an n- semiconductor (more precisely an intrinsic semiconductor) which is assigned to the compound semiconductors. Below 230 ° C, magnesium silicide shows a brittle material behavior, above it becomes plastic. The breaking strength of the single crystals decreases with increasing temperature from 100 MPa at room temperature to 10 MPa at 630 ° C. It is resistant to alkalis, acids, on the other hand, decompose it with the formation of silicon hydrides ( silanes ) and hydrogen. Magnesium chloride and monosilane are formed on contact with hydrochloric acid .
use
Magnesium silicide is used to manufacture silanes and to improve the properties of magnesium and aluminum alloys . In aluminum alloys, they increase strength through precipitation hardening ; They can therefore first be processed in the soft state and only then hardened by a heat treatment. The most important alloy groups are the aluminum-silicon alloys and the aluminum-magnesium-silicon alloys .
Individual evidence
- ↑ a b c d Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 916.
- ↑ a b c d e data sheet Magnesium silicide, ≥99% trace metals basis, −20 mesh from Sigma-Aldrich , accessed on August 23, 2012 ( PDF ).
- ↑ American elements: Magnesium Silicide
- ↑ a b c d Nikolaus Reinfried; Modification of the materials based on magnesium silicide with the help of Spark plasma synthesis, March 15, 2006, urn : nbn: de: swb: 14-1176195464803-87414
- ↑ Kevin Hutchings: Classic Chemistry Experiments . Royal Society of Chemistry, 2000, ISBN 0-85404-919-3 , pp. 181 ( limited preview in Google Book search).
- ^ Inorganic chemistry. In: Journal of the Chemical Society, Abstracts. 94, 1908, pp. B172-B200, doi : 10.1039 / CA9089405172 , here: p. B184.
- ↑ J. Tani, M. Takahashi, H. Kido: Thermoelectric properties and oxidation behavior of Magnesium Silicide. In: IOP Conference Series: Materials Science and Engineering. 18, 2011, p. 142013, doi : 10.1088 / 1757-899X / 18/14/142013 .
- ↑ Seilnacht: Periodic Table: Silicium
- ^ Edward Ghali: Corrosion Resistance of Aluminum and Magnesium Alloys: Understanding ... John Wiley & Sons, 2010, ISBN 0-470-53176-2 , pp. 139 ( limited preview in Google Book search).


