magnesium
| properties | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Magnesium, Mg, 12 | ||||||||||||||||||||||||||||||||||||||||||
| Element category | Alkaline earth metals | ||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 2 , 3 , p | ||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white | ||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7439-95-4 | ||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-104-6 | ||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.276 | ||||||||||||||||||||||||||||||||||||||||||
| ATC code | |||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 1.94% | ||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 24.305 (24.304-24.307) u | ||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 150 (145) pm | ||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 141 pm | ||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 173 pm | ||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Ne ] 3 s 2 | ||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 7th.646 236 (4) eV ≈ 737.75 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 15th.035 271 (6) eV ≈ 1 450.68 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 80.1436 (6) eV ≈ 7 732.68 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 109.2654 (12) eV ≈ 10 542.51 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 141.33 (3) eV ≈ 13 636 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal | ||||||||||||||||||||||||||||||||||||||||||
| density | 1.738 g / cm³ (20 ° C ) | ||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.5 | ||||||||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( Χ m = 1.2 · 10 −5 ) | ||||||||||||||||||||||||||||||||||||||||||
| Melting point | 923 K (650 ° C) | ||||||||||||||||||||||||||||||||||||||||||
| boiling point | 1383 K (1110 ° C) | ||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 14.00 10 −6 m 3 mol −1 | ||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 132 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 8.7 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 4602 m s −1 at 293.15 K. | ||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 1023 J kg −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||
| Work function | 3.66 eV | ||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 22.7 · 10 6 A · V −1 · m −1 | ||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 160 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 1, 2 | ||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −2.372 V (Mg 2+ + 2 e - → Mg) | ||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.31 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||
Magnesium is a chemical element with the element symbol Mg (alchemy: ⚩) and the atomic number 12. In the periodic table of the elements it is in the second main group or the 2nd IUPAC group and thus belongs to the alkaline earth metals .
Magnesium is one of the ten most common elements in the earth's crust . It occurs in numerous minerals as well as in the leaf green of plants.
history

The origin of the element designation is presented differently in the literature:
- from ancient Greek μαγνησία λίθος meaning "magnetic stone",
- of Magnisia , an area in eastern Greece ,
- from Magnesia , a city in Asia Minor in what is now Turkey.
However, all of the given derivations seem etymologically to come from the magnets or their eponymous hero Magnes .
Magnesium compounds were known and in use centuries before the production of elemental magnesium. Magnesia alba referred to magnesium carbonate , while magnesia was the common name for magnesium oxide .
The Scottish physicist and chemist Joseph Black was the first to systematically study magnesium compounds in the 18th century. In 1755 he recognized in his work De humore acido a cibis orto et Magnesia alba the difference between lime ( calcium carbonate ) and magnesia alba (magnesium carbonate), which were often confused at that time. He understood Magnesia alba as the carbonate of a new element. This is why Black is often cited as the discoverer of magnesium, although he never represented elemental magnesium.
In 1808 Sir Humphry Davy obtained magnesium by electrolysis of moistened magnesium hydroxide with the help of a voltaic ash column - not in pure form, but as an amalgam , as he worked with a cathode made of mercury . He showed that magnesia is the oxide of a new metal that he initially called magnium .
In 1828 the French chemist Antoine Bussy was able to produce small amounts of pure magnesium by heating dry magnesium chloride with potassium as a reducing agent. In 1833, Michael Faraday was the first to produce magnesium through the electrolysis of molten magnesium chloride. Based on these experiments, the German chemist Robert Wilhelm Bunsen worked in the 1840s and 1850s on processes for the production of magnesium by electrolysis of molten salts with the help of the Bunsen element he developed . In 1852 he developed an electrolysis cell for the production of large quantities of magnesium from molten, anhydrous magnesium chloride. This method has been preferred to this day for the production of magnesium.
The technical production of magnesium began in France in 1857 using a process by Henri Etienne Sainte-Claire Deville and H. Caron. In the so-called Deville-Caron process, a mixture of anhydrous magnesium chloride and calcium fluoride is reduced with sodium . In England , Johnson Matthey began producing magnesium using a similar process around 1860. However, due to manufacturing difficulties, these early ventures remained uneconomical.
Occurrence

Magnesium does not occur in elemental form in nature because of its responsiveness. As a mineral , it occurs mainly in the form of carbonates , silicates , chlorides and sulfates . In the form of dolomite , a magnesium mineral is even mountain-forming, e.g. B. in the Dolomites .
The most important minerals are dolomite CaMg (CO 3 ) 2 , magnesite (bitter spar) MgCO 3 , olivine (Mg, Fe) 2 [SiO 4 ], enstatite MgSiO 3 and kieserite MgSO 4 · H 2 O.
Other minerals are:
- Serpentine Mg 3 [Si 2 O 5 ] (OH) 4
- Talc Mg 3 [Si 4 O 10 ] (OH) 2
- Sepiolite Mg 4 [Si 6 O 15 ] (OH) 2
- Schönite K 2 Mg (SO 4 ) 2 · 6 H 2 O
- Carnallite KMgCl 3 · 6H 2 O
- Spinel MgAl 2 O 4
When dissolved in water, together with calcium it causes water hardness . In seawater , it is more than 1 kg / m³ included.
Extraction and presentation
There are two main ways in which magnesium is obtained:
- By fused- salt electrolysis of molten magnesium chloride in Downs cells : Downs cells consist of large iron troughs that are heated from below. Graphite rods embedded from above serve as anodes , which are surrounded at the tips by a ring-shaped cathode . The metallic magnesium collects on the molten salt and is skimmed off. The resulting chlorine gas collects in the upper part of the cell and is used again to produce magnesium chloride from magnesium oxide . Calcium and sodium chloride are added to the molten salt to lower the melting point of the magnesium chloride.
- By thermal reduction of magnesium oxide (Pidgeon process): Burnt dolomite , barite and a reducing agent such as ferrosilicon are poured into a container made of chrome - nickel - steel . It is then evacuated (pumping off the gas ) and heated to 1160 ° C. The vaporous magnesium condenses on the water-cooled head piece outside the furnace . The magnesium obtained in batches is further purified by vacuum distillation .
The Pidgeon process is the most important manufacturing process today and is mainly used in China.
88% of global magnesium production takes place in China, where around 800,000 t of magnesium metal were produced in 2015. This is followed by Russia , Israel and Kazakhstan, each with a market share of just a few percent .
The production of 1 kg of magnesium by the Pidgeon process produces greenhouse gases with a CO 2 equivalent of around 31 kg (for comparison: 1 kg of steel produces between 0.5 and 2 kg of CO 2 equivalents).
Although magnesium is found in more than 60 minerals, only dolomite , magnesite , brucite , carnallite , talc and olivine are of commercial importance.
The Mg 2+ - cation is in seawater second most abundant cation, which sea water and sea salt makes them attractive commercial sources of magnesium. To extract it, calcium hydroxide is added to sea water to form a precipitate of magnesium hydroxide .
Magnesium hydroxide (brucite) is insoluble in water and can be filtered off and reacted with hydrochloric acid to form concentrated magnesium chloride.
By electrolysis arises from magnesium chloride magnesium.
properties
The solid, silvery, shiny light metal magnesium is around a third lighter than aluminum . Pure magnesium is poor in strength and hardness. Its modulus of elasticity is around 45 GPa. In air, magnesium is covered with an oxide layer that, unlike aluminum, is not completely opaque. The reason for this is that the magnesium oxide has a smaller molar volume than magnesium itself (MgO: 10.96 cm 3 / mol, Mg: 13.96 cm 3 / mol); s. Pilling-Bedworth ratio .
Thin ribbons or foils can be easily ignited. It burns in the air with a bright white flame to form magnesium oxide MgO and a little magnesium nitride Mg 3 N 2 . Freshly made magnesium powder can heat up in the air and even ignite. Dangerous reactions are to be expected at higher temperatures, i.e. especially with molten liquids. Magnesium also burns in many oxides such as carbon monoxide , nitrogen oxide and sulfur dioxide .
Magnesium reacts with water to form hydrogen:
- Reaction of magnesium with water
A sparingly soluble coating of magnesium hydroxide forms , which largely brings the reaction to a standstill ( passivation ). Even weak acids , such as ammonium salts , are sufficient to dissolve the hydroxide layer, as they convert the hydroxide ions into water and soluble salts are formed. Without passivation, the exothermic reaction is violent; the finer the magnesium dust, the more violent it is. The released hydrogen easily forms an explosive mixture with air ( oxyhydrogen ).
Magnesium reacts exothermically with carbon dioxide to form magnesium oxide and carbon:
Reaction of magnesium with carbon dioxide
Therefore, carbon dioxide does not extinguish magnesium fires, but rather fuels them.
In contrast to aluminum, it is relatively resistant to hydrofluoric acid and bases . The reason for this is the low solubility of the magnesium fluoride (MgF 2 ) formed as a coating, which prevents further formation of Mg (OH) 3 - ions.
Isotopes
A total of 21 isotopes between 19 mg and 40 mg of magnesium are known. Of these three, the isotopes 24 mg, 25 mg and 26 mg are stable and occur in nature. The isotope with the greater proportion of the natural isotopic composition is 24 mg with 78.99%, 25 mg has a proportion of 10.0% and 26 mg of 11.01%. The longest-lived unstable isotopes are 28 Mg, which converts to 28 Al with a half-life of 20.915 hours with beta decay , and 27 Mg, which also decays to 27 Al with a half-life of 9.435 minutes with beta decay. All other isotopes only have short half-lives of seconds or milliseconds.
use
Metallic magnesium
Magnesium powder and wire are used in incendiary devices , bombs and light ammunition, and in former times also used as flashlight powder . Magnesium rods often serve as sacrificial anodes , which protect parts made of noble metals from corrosion .
In metallurgy magnesium is used versatilely,
- z. B. as a reducing agent in the Kroll process for the extraction of titanium ,
- as a reducing agent for the extraction of uranium , copper , nickel , chromium and zirconium ,
- as a component of aluminum alloys of the groups AlSiMg and AlMg,
- as magnesium granulate for desulphurisation of iron and steel ,
- as an additive for spheroidal graphite cast iron
Magnesium is based on a group of standardized light alloys for the construction of aircraft and motor vehicles (which melting require a covering layer of molten magnesium chloride to protect against exposure to air and oxidation, s. Melt treatment ), see also electron (Material)
Another application is torches that burn underwater.
In organic chemistry , it is used to produce Grignard compounds .
Because magnesium ignites very easily, it is also used as a lighter that works even under adverse conditions. The magnesium blocks sold as Fire Starter Kits are supplied with a flint , the abrasion of which ignites spontaneously in the air. The procedure is similar to the Stone Age method of lighting a fire with flint and tinder , with magnesium taking on the role of tinder. First, chips are scraped off the magnesium block with a knife and placed on or under the actual fuel. Then, by scraping the flint (e.g. with the back of the knife), sparks are created as close as possible to the magnesium chips in order to ignite them.
Magnesium alloys
The most important property of magnesium alloys, which has made them important compared to aluminum and its alloys, is the lightweight construction they make possible. With a density of around 1.75 g / cm³, the difference to lightweight aluminum construction with a density of around 2.75 g / cm³ is clear. In addition, the melting range is between 430 and 630 ° C, i.e. lower to save energy. However, the mechanical properties such as tensile strength and hardness are significantly lower than those of aluminum alloys . The low density made magnesium interesting for mobile applications early on. The first large-scale application took place before the First World War in the construction of the scaffolding for the rigid Zeppelin airships . Magnesium alloys were used in motor vehicles for the production of housing parts and for the production of wheel rims for all kinds of mobiles. After 1930, magnesium alloys were increasingly used in aircraft construction, because the weight savings they made allowed more energy-efficient flights as well as higher payloads. All of this led to a rapid expansion of magnesium production in Germany ( electron from the chemical factory in Griesheim ) and, after 1940, also in the USA. Immediately after production started, "Elektron" became the trademarked name for the first magnesium alloys.
Other possible uses for magnesium castings offered themselves in the course of technical developments, partly due to the war, partly constructively foresighted and at the same time optimizing the alloys. The alloys Mg-Al, Mg-Mn, Mg-Si, Mg-Zn and finally Mg-Al-Zn alloys were developed as magnesium-based materials.

The gearbox of the VW Beetle was cast in millions from a Mg-Si alloy. Today magnesium alloys are not only used to save weight, but are also characterized by high damping. When exposed to vibrations, this leads to a reduction in vibration and noise emissions. For this reason, too, magnesium alloys have become interesting materials in engine construction and in automobile construction in general. So parts are not only the engine of magnesium alloy, but increasingly also for casting engine blocks hybrid process / hybrid cast applied for the first time in volume production in the Alfa Romeo 156 and later at BMW (see also BMW N52 ).

In the die casting process (see also under die casting ), many, even large, thin-walled components can be produced close to the final dimensions and without costly post-processing. B. rims, profiles, housings, doors, hoods, trunk lids, handbrake levers and others. Parts made of Mg-Al-Zn alloys are not only used in automotive engineering, but also in mechanical engineering.
Efforts towards lightweight construction already led to magnesium-lithium alloys at the end of the 20th century , even lighter alloys made from magnesium with the addition of lithium .
Magnesium materials in medicine
The latest research promises a high development potential of magnesium materials as absorbable implant material (e.g. as a stent ) for the human body. Magnesium materials must be protected from contact corrosion during use. The corrosion resistance to normal atmospheric influences, however, is good. The contact corrosion behavior would be a decisive advantage if it were used as an implant material to be used for a limited period of time, since it would dissolve safely after a certain time. This eliminates the risks and costs of an operation to remove implants.
physiology
Magnesium is one of the essential substances and is therefore indispensable for all organisms. The green leaves of plants, the chlorophyll , contain around 2% magnesium. There it forms the central atom of chlorophyll. In magnesium deficiency etiolate plants as well as with lack of light. The human body must also be supplied with sufficient amounts of magnesium on a daily basis in order to prevent a magnesium deficiency.
An adult's body contains around 20 g of magnesium (for comparison: 1000 g of calcium ). In the blood plasma, 40% of the magnesium is bound to proteins; the normal serum level is 0.8–1.1 mmol / l. Magnesium is involved in around 300 enzyme reactions as an enzyme component or coenzyme. In addition, free Mg ions influence the potential on the cell membrane and act as a second messenger in the immune system . They stabilize the resting potential of excitable muscle and nerve cells and the cells of the autonomic nervous system . Magnesium deficiency triggers restlessness, nervousness, irritability, lack of concentration, tiredness, general weakness, headaches , cardiac arrhythmias and muscle cramps . Heart attacks can also occur. In the area of metabolism and psyche, it is assumed that magnesium deficiency exacerbates depression and schizophrenic psychoses . An excess of magnesium in the blood can result from excessive intake and kidney dysfunction and leads to disorders in the nervous system and heart.
The Magnesiumresorption first found in the upper small intestine instead of, but in the rest of the digestive tract. It is excreted through the kidneys and is contained in different amounts in all foods and in drinking water. The required daily dose of around 300 mg is usually achieved through a balanced diet. An increased need can be met with dietary supplements or medication . A slight magnesium deficiency is possible due to serious illness, pregnancy or competitive sports. Severe deficiencies occur with kidney dysfunction, long-term diarrhea, chronic intestinal inflammation, poorly controlled diabetes mellitus, corticoids , certain diuretics or alcoholism with malnutrition.
Magnesium salts such as citrate , gluconate , aspartate and aspartate hydrochloride are approved as medicinal products in Germany, namely in daily doses of 100 to 400 mg against deficiencies and neuromuscular disorders such as muscle cramps, migraines or pregnancy complications . Side effects are gastrointestinal complaints and diarrhea, in the event of an overdose also fatigue and slow pulse. Renal dysfunction and certain cardiac arrhythmias are contraindications .
If magnesium supplements are taken orally (tablets, chewable or lozenges, granules for dissolving in liquid), the dosage is important. Various studies have come to the conclusion that around 35% is absorbed when taking 120 mg , but only around 18% when taking a complete daily dose of 360 mg. For resorption in the body, the form of the compounds used today in drugs is irrelevant, because they are pharmacologically, biologically and clinically equivalent; Organic salts such as magnesium aspartate or magnesium citrate are merely absorbed by the body more quickly than inorganic compounds. On the other hand, the additional magnesium only remains usefully in the body if there are enough binding molecules available in the body; this happens through biochemical adjustments only after a longer increase in the magnesium supply or consumption for at least four weeks.
Magnesium sulfate ("Epsom salt") was previously used as a laxative.
Magnesium salts are used in alternative medicine .
Food
Magnesium serves as a cofactor for around 300 different proteins , especially in ATP and nucleic acid binding enzymes . The recommended daily intake of magnesium for humans is between 24 and 400 mg per day, depending on age and gender.
Magnesium occurs as a compound in many foods, especially in whole grain products (for example whole grain bread , whole grain pasta , whole grain rice , oat flakes , cornflakes ), mineral water , especially medicinal water , tap water with sufficient water hardness , liver , poultry , table fish , pumpkin seeds , sunflower seeds , chocolate , Cashews , peanuts , potatoes , spinach , kohlrabi , soft fruits , oranges , bananas , sesame seeds , beet syrup , milk and dairy products .
Hazards and protective measures
The dangerousness of elemental magnesium depends strongly on the temperature and the particle size: compact magnesium is harmless at temperatures below the melting point, while magnesium chips and powder are highly flammable . Due to the large surface area, the latter can easily react with the oxygen in the air. With very fine magnesium powder there is a risk of spontaneous combustion ; Air-powder mixtures are even explosive . Phlegmatization is a risk-reducing treatment when processing magnesium and metal powders. Molten magnesium also self-ignites in air. Fine-grained or heated magnesium also reacts with many other substances, for example water and other oxygen-containing compounds. Magnesium melts therefore require permanent protection against the ingress of atmospheric oxygen. In practice, this is done by covering the melt with agents rich in magnesium chloride. Sulfur hexafluoride is also suitable as protection against oxidation. Covering with elemental sulfur, which was customary in the past, is no longer practiced because of the severe nuisance caused by the sulfur dioxide that is produced.
Temperatures of up to around 3000 ° C occur in magnesium fires. Under no circumstances must common extinguishing agents such as water , carbon dioxide , foam or nitrogen be used, as magnesium reacts violently with these. If water comes into contact with a magnesium fire, there is an acute risk of an oxyhydrogen reaction .
For the fire ( metal fires ) of a melt, the extinguishing principle of suffocation applies, i.e. the rapid displacement of oxygen. In the simplest case by covering with dry sand, otherwise by applying a covering salt for magnesium melts. Extinguishing powders of fire class D, magnesium oxide powder ( Magnesia usta / burned magnesia ) and, if necessary, dry rust-free gray cast iron chips are also suitable .
When using magnesium, all given safety instructions must be followed exactly. Under no circumstances may an explosive atmosphere (magnesium dust, hydrogen, aerosols and vapors of flammable cooling lubricants) arise. The normal occupational safety measures , such as avoiding ignition sources , must also be observed.
proof
The best way to detect magnesium is using Magneson II , titanium yellow or quinalizarin .
For detection with Magneson II (4- (4-nitrophenylazo) -1-naphthol), the original substance is dissolved in water and made alkaline. Then a few drops of a solution of the azo dye Magneson II are added. If magnesium ions are present, a dark blue colored lacquer is created. Other alkaline earth metals should first be removed as carbonates by precipitation.
For detection with titanium yellow ( thiazole yellow G), the original substance is dissolved in water and acidified. Then it is mixed with a drop of the titanium yellow solution and made alkaline with dilute sodium hydroxide solution. If magnesium is present, a light red precipitate is formed. Nickel, zinc, manganese and cobalt ions interfere with this detection and should be precipitated as sulfides beforehand.
For detection with quinalizarin, two drops of the dye solution are added to the acidic sample solution. Then dilute sodium hydroxide solution is added until the reaction is basic. A blue color or precipitation indicates magnesium.
The formation of precipitates with phosphate salt solutions can also be used as a detection reaction for magnesium salts. The heavy metal-free sample solution, which is buffered to pH 8 to 9 with ammonia and ammonium chloride, is mixed with disodium hydrogen phosphate solution. A white, acid-soluble cloudiness caused by magnesium ammonium phosphate MgNH 4 PO 4 indicates magnesium ions:
From ammoniacal solution, Mg 2+ can also be detected with oxine as a poorly soluble yellow-greenish compound. This proof is suitable for the cation separation process .
links
Magnesium occurs almost exclusively in compounds as a divalent cation with the oxidation state 2.
Oxides and hydroxides
Magnesium oxide (magnesia) forms colorless crystals of sodium chloride - structure . In nature it occurs as a volcanic mineral periclase . They are white to gray, due to inclusions also dark green, glass-shining regular crystals.
Magnesia powder is added to foods as an acidity regulator or release agent. Various heat-resistant objects for laboratories and industry are made from magnesium oxide ceramics .
Magnesium hydroxide is a colorless, strongly basic salt and occurs naturally as the mineral brucite . It has a trigonal crystal structure in the space group P 3 m 1 (space group no. 164) and is used as a cooking oil additive (for binding sulfur dioxide ), as a flocculant for waste water treatment , as flame retardants in thermoplastic plastics ( polyolefins , polyvinyl chloride ) and elastomers , as well as Additive used in detergents . In medicine it is used as an antacid to neutralize the stomach acid and as a mild laxative used.
Magnesium peroxide is a pulverulent, colorless compound having a pyrite - crystal structure in the space group Pa 3 (space group # 205.) . It is similar to calcium peroxide and releases oxygen through a controlled reaction with aqueous solutions . It has various uses in agriculture , pharmaceuticals and cosmetics .
fertilizer
When liming arable and grassland areas, magnesium is used in the form of magnesium oxide or magnesium carbonate to compensate for the magnesium depletion of the soil by the plants . Furthermore, the soil pH value is increased and the availability of further nutrients is improved. Here, the magnesium compound is mostly used together with lime as a complex fertilizer containing magnesium and calcium . Magnesium phosphate Mg 3 (PO 4 ) 2 ( trimagnesium phosphate ), which occurs naturally as Bobierrite, and magnesium nitrate are also used as complex fertilizers.
Halides

Magnesium chloride is highly hygroscopic and occurs naturally in the mineral bishopite (MgCl 2 · 6 H 2 O), as the double salt carnallite (KMgCl 3 · 6 H 2 O), in sea water and in salt lakes . It crystallizes in the trigonal crystal system in the space group R 3 m (space group no. 166) . In food technology, it is used as an acid regulator , firming agent , flavor enhancer , carrier or separating agent. As a thermal battery, magnesium chloride hexahydrate can store and release heat energy .
Magnesium fluoride forms colorless crystals which tetragonal in the rutile structure in the space group P 4 2 / mnm (space group no. 136) space group crystallize . Its optical properties, together with its chemical stability, make it an important material for optical applications.
Magnesium bromide and magnesium iodide are also hygroscopic salts which have a trigonal crystal structure in the space group P 3 m 1 (space group no. 164) .
Other inorganic compounds
Magnesium carbonate occurs naturally in large quantities as magnesite (bitter spar). It crystallizes trigonally in the space group R 3 c (space group no. 167) . In the food industry it is added as an acid regulator , carrier or separating agent. It is used in climbing and gymnastics and is also known under the names magnesia and chalk . The athletes then dry in it before the beginning of the exercise the palms so that her skin while covering the bars of bullion or the iron bars of stretching or long dumbbell not too liable. It also has medical and industrial uses.
Magnesium nitrate is a colorless, hygroscopic salt that is easily soluble in water . The hexahydrate (Mg (NO 3 ) 2 · 6 H 2 O) has a monoclinic crystal structure with the space group P 2 1 / c (space group no. 14) . It is used as fertilizer , latent heat storage (as hexahydrate) or in the ceramic industry.
Magnesium sulfate heptahydrate (Mg (SO 4 ) 7H 2 O) is known as the mineral epsomite (Epsom salt). It forms colorless crystals that form a rhombic pseudotetragonal crystal lattice . The crystals often bloom in fibrous aggregates and form stalactites . It is used for fertilizers , as a desiccant and for medicinal applications.
Magnesium phosphates (magnesium dihydrogen phosphate (Mg (H 2 PO 4 ) 2 ), magnesium hydrogen phosphate (MgHPO 4 ) and magnesium phosphate (Mg 3 (PO 4 ) 2 )) are used in industry as ceramic raw materials and as flame retardants . In the food industry , they are used as a feed additive , laxative and food additive . They are added to food as an acidity regulator or release agent.
Spinel is a frequently occurring mineral from the mineral class of oxides and hydroxides with the idealized chemical composition MgAl 2 O 4 and is therefore, chemically speaking, a magnesium aluminate . It crystallizes isotypically with magnetite in the cubic crystal system in the space group Fd 3 m (space group no. 227) .
Dolomite is a very common mineral from the mineral class of carbonates and nitrates with the chemical composition CaMg [CO 3 ] 2 and is therefore, chemically speaking, a calcium- magnesium carbonate . It crystallizes in the trigonal crystal system in the space group R 3 (space group no. 148) .
Magnesium hydride can be used to store hydrogen and energy . By hydrogen , which is released from magnesium hydride, a metal foam with interesting properties, which lighter than water , is generated.
Further interesting crystalline magnesium compounds are, for example, magnesium diboride , magnesium carbide , magnesium nitride , magnesium sulfide , magnesium silicide , magnesium germanide , magnesium metasilicate , magnesium titanium oxide and magnesium polonide .
Magnesium organyls
Magnesium organyls are organometallic compounds in which there is a bond between magnesium and carbon . Among the magnesium organyls , Grignard compounds ( R -Mg- X ) are by far the most important. Binary organyls of magnesium and alkenyl magnesium halides play a clearly subordinate role.
Organyl magnesium halides
Organyl magnesium halides (usually called Grignard compounds ) are obtained in a direct process through the reaction of organyl halides with magnesium shavings. Grignard reagents are in solution in the Schlenk equilibrium . They react with halogen - organyl - substitution to element organyls:
General:
z. B.:
or with the addition of organyls with multiple bond systems :
General:
z. B .:
Binary magnesium organyls
Binary magnesium organyls (R 2 Mg, also called magnesium diorganyls) can be produced in different ways:
- through transmetalation , for example of mercury diorganyls:
- by dismutation in the shift of the Schlenk equilibrium with the help of 1,4-dioxane :
- Magnesacycles ( cyclic alkanes with a magnesium in the ring) can also be prepared with the aid of 1,4-dioxane .
- by metathesis of Grignard compounds with lithium organyls
- by hydromagnesation (addition of MgH 2 to 1-alkenes):
- due to the addition of elemental magnesium to C = C double bonds in some unsaturated hydrocarbons such as 1,3-butadiene or anthracene (metal addition). For example, the reaction of 1,3-butadiene in tetrahydrofuran at room temperature is possible :
- The magnesium-butadiene produced, and (2-butene-1,4-diyl) magnesium mentioned, can be used as a source of butadiene - anions in further syntheses are used. The orange-yellow magnesium anthracene is displayed analogously . Magnesium anthracene can then be used as a catalyst for the hydrogenation of magnesium.
Alkenyl magnesium halides
In what is known as carbomagnesation, alkynes react with alkynes to form alkenyl magnesium halides:
Other organic compounds
Magnesium hydrogen citrate and trimagnesium dicitrate are magnesium salts of citric acid . Magnesium citrate is used as a medicine .
Magnesium monoperoxyphthalate is a disinfectant for surface disinfection .
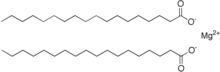
Magnesium stearate is the magnesium salt of stearic acid and is one of the lime soaps . It consists of a magnesium ion and two long-chain stearate ions.
Web links
Individual evidence
- ↑ a b Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (magnesium) , unless otherwise stated .
- ↑ IUPAC, the standard value recommended by IUPAC is given, since the isotopic composition of this element can vary locally, the mass range given in brackets results for the mean atomic weight. Standard Atomic Weights Revised 2013 .
- ↑ a b c d e Entry on magnesium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e entry on magnesium at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 136.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Magnetic Susceptibility of the Elements and Inorganic Compounds, pp. 4-144. The values there are based on g / mol and are given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data. Volume 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ Ludwig Bergmann, Clemens Schaefer, Rainer Kassing: Textbook of Experimental Physics. Volume 6: Solids. 2nd Edition. Walter de Gruyter, 2005, ISBN 3-11-017485-5 , p. 361.
- ↑ A. Stasch, C. Jones: Stable dimeric magnesium (I) compounds: from chemical landmarks to versatile reagents. In: Dalton Transactions . Volume 40, 2011, pp. 5659-5672, doi: 10.1039 / C0DT01831G .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Electrochemical Series, pp. 8-22.
- ↑ a b Entry on magnesium, powder, not stabilized in the GESTIS substance database of the IFA , accessed on August 9, 2016 (JavaScript required)
- ↑ Entry on magnesium in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Definition of characters 26A9 (Hex) as magnesium in the Unicode Standard, Version 5.2 (PDF; 291 kB).
- ^ US Geological Survey, Mineral Commodity Summaries, January 2016
- ↑ Volker Hasenberg: Life cycle analysis and ecological evaluation of magnesium production. PE INTERNATIONAL, Materials Forum for Intelligent Lightweight Trees April 24, 2012, Hanover Fair.
- ↑ The Reaction Between Magnesium and CO 2 . Purdue University, accessed June 15, 2016 .
- ↑ G. Audi, FG Kondev, Meng Wang, WJ Huang, S. Naimi: The NUBASE2016 evaluation of nuclear properties. In: Chinese Physics C. 41, 2017, S. 030001, doi: 10.1088 / 1674-1137 / 41/3/030001 (full text)
- ↑ FY Li, B. Chaigne-Delalande include: second messenger role for Mg 2+ revealed by human T-cell immunodeficiency. In: Nature. Volume 475, Number 7357, July 2011, pp. 471-476; doi: 10.1038 / nature10246 . PMID 21796205 ; PMC 3159560 (free full text).
- ↑ a b c Sighart Golf: Bioavailability of organic and inorganic compounds. In: Pharmaceutical newspaper . July 2009, accessed March 4, 2015 .
- ^ R. Swaminathan: Magnesium metabolism and its disorders. In: Clin Biochem Rev. 24 (2), May 2003, pp. 47-66. PMID 18568054 .
- ↑ z. B. Kenneth D Fine, Carol A Santa Ana, Jack L Porter, John S Fordtran: Intestinal absorption of magnesium from food and supplements. In: J Clin Invest . Volume 88 (2), August 1991, pp. 396-402, PMC 295344 (free full text, PDF), doi: 10.1172 / JCI115317 .
- ^ German Society for Nutrition : The reference values for nutrient intake: Magnesium . Retrieved October 20, 2013.
- ↑ Entry on magnesium hydroxide. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 907.
- ↑ Liming of arable land and grassland. (PDF; 43 kB) (No longer available online.) DLR Rheinhessen-Nahe-Hunsrück, archived from the original on February 14, 2016 ; accessed on February 14, 2016 .
- ↑ Science-Online-Lexica: Magnesiumphosphate. In: Lexicon of Chemistry. Retrieved May 26, 2011.
- ↑ Entry on magnesium nitrate. In: Römpp Online . Georg Thieme Verlag, accessed on May 26, 2011.
- ↑ Heat storage from salt, article from 2012
- ↑ Entry for CAS no. 7783-40-6 in the GESTIS substance database of the IFA , accessed on October 17, 2012(JavaScript required) .
- ^ MA Brogan, AJ Blake, C. Wilson, DH Gregory: Magnesium diiodide, MgI 2 . In: Acta Crystallographica , C59, 2003, pp. I136-i138, doi: 10.1107 / S0108270103025769 .
- ^ Hans Lohninger: Magnesia for athletes. Retrieved July 6, 2019 .
- ↑ Entry on magnesium nitrate in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Spinel. In: mindat.org. Hudson Institute of Mineralogy, accessed May 15, 2019 .
- ^ Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 188 .
- ^ Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 287 .
- ^ Reuben D. Rieke: Preparation of highly reactive metal powders and their use in organic and organometallic synthesis. In: Accounts of Chemical Research. Volume 10, Number 8, August 1977, pp. 301-306, doi: 10.1021 / ar50116a005 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1233.
- ↑ Christoph Elschenbroich : Organometallchemie . 6th, revised edition. Vieweg + Teubner, 2008, ISBN 978-3-519-53501-0 ( book preview in the Google book search).
- ↑ Yukitami Saheki, Katsuhiko Sasada, Nobumasa Satoh, Noriyuki Kawaichi, Kenji Negoro: A Convenient Preparation of Pure Dialkylmagnesium from a Grignard Reagent. In: Chemistry Letters. Volume 16, 1987, pp. 2299-2300, doi: 10.1246 / cl.1987.2299
- ↑ Borislav Bogdanovic: Magnesium anthracene systems and their application in synthesis and catalysis. In: Accounts of chemical research. Volume 21, Number 7, July 1988, pp. 261-267, doi: 10.1021 / ar00151a002 .
- ↑ K. Fujita, Y. Ohnuma, H. Yasuda, H. Tani: Magnesium-butadiene addition compounds: Isolation, structural analysis and chemical reactivity. In: Journal of Organometallic Chemistry. Volume 113, Number 3, June 1976, pp. 201-213, doi: 10.1016 / S0022-328X (00) 87329-5 .
- ^ Ramsden, HE 63: US Patent 1967, 3, 354, 190.
- ↑ Entry on organic magnesium compounds. In: Römpp Online . Georg Thieme Verlag, accessed on May 26, 2014.
- ↑ Franz v. Bruchhausen, Siegfried Ebel, Eberhard Hackenthal, Ulrike Holzgrabe: Hager's Handbook of Pharmaceutical Practice, Volume 5: Substances LZ . Springer-Verlag, 2013, ISBN 978-3-642-58388-9 , pp. 86 ( limited preview in Google Book search).