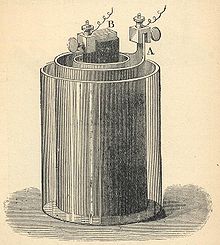
Bunsen element
The Bunsen element is a voltage source presented by Robert Bunsen in 1841 , which supplies an electrical voltage of around 1.9 V. It was used for scientific and technical experiments and applications, such as electrical lighting, until at least the mid-1880s, and is therefore historically significant. It is a galvanic element that was often used as a form of battery , and an improved variant of the Grove element developed by William Grove in 1839 .
The element of Grove consisted of a zinc - electrode , in sulfuric acid as electrolyte appeared surrounded by a porous clay cylinder as a diaphragm ; platinum in nitric acid served as the counter electrode . Bunsen succeeded in replacing the expensive platinum electrode with a cylinder made of much cheaper pressed carbon . The Bunsen element was one of the most powerful sources of electricity in its time.
The comparatively large currents that could be achieved with it were made possible by the relatively large surface area of the carbon used. In addition, Bunsen dispensed with a diaphragm ("whereby the porous clay cells necessary for constant batteries are dispensable"), so that the internal resistance of his cell was also low. The disadvantage was that the zinc corroded very quickly: Johann Christian Poggendorff tried a replica of the Bunsen cell and reported such a "violent dissolution of the zinc" that he took the device apart after a quarter of an hour. In addition, the toxic and corrosive gases and vapors of nitric acid and the escaping nitrogen oxides are disadvantageous. The glass vessels were therefore designed in such a way that they restricted the escape of vapors; later closed vessels were also used.
Important uses of the bunion cell
In Bunsen's laboratory his zinc-carbon cells were e.g. B. for the production of chlorine oxyhydrogen and for the representation of elements, including lithium , aluminum and sodium . Hermann Kolbe also used them in Bunsen's laboratory in Marburg in his experiments on electrolysis, which led to the discovery of the reaction known today as Kolbe electrolysis . In 1886, Henri Moissan used a battery of 50 buns cells for the first presentation of elemental fluorine .
In 1841, on the Quai de Conti in Paris, the first attempts were made to electrically illuminate a public square, using a battery of 100 Bunsen elements. In 1846 the Paris Opera was equipped with arc lamps powered by a battery of 360 Bunsen elements.
Reaction equations
At the anode (negative pole, the zinc sheet, see zinc electrode ) of the Bunsen cell, the zinc dissolves when discharging, it is oxidized to the zinc ion:
- .
The nitric acid HN V O 3 is reduced at the cathode of the cell (positive pole, the carbon electrode soaked with nitric acid) . Depending on the reaction conditions or how complete the reaction is, nitrogen dioxide N IV O 2 , nitrite N III O 2 - or - in the case of extensive discharge - nitrogen monoxide N II O is produced. With concentrated nitric acid and limited depth of discharge, nitrogen dioxide is produced:
The overall reaction of the cell (see) is therefore:
- .
The voltage of the cell is between 1.888 V and 1.964 V.
Web links
Individual evidence
- ^ A b Robert Bunsen: About a new construction of the galvanic column . In: Friedrich Wöhler, Justus Liebig (Ed.): Annals of Chemistry and Pharmacy . tape 38 , no. 3 . CF Winter, Heidelberg 1841, p. 311–313 , doi : 10.1002 / jlac.18410380307 ( online at the Internet Archive (in the collected works); online at the HathiTrust Digital Library - the work is dated May 14, 1841.).
- ↑ Robert Bunsen: About the application of coal to voltaic batteries . In: Johann Christian Poggendorff (Ed.): Annals of Physics and Chemistry . 130 (Pogg. Ann. 54), no. 11 . Johann Ambrosius Barth, Leipzig 1841, p. 417-430 , doi : 10.1002 / andp.18411301109 ( online at Gallica , Bibliothèque nationale de France).
- ↑ a b Bunsen's improved carbon battery and some experiments with the same . In: Annals of Physics and Chemistry . 136 (Pogg. Ann. 60), no. 11 , 1843, p. 402–405 , doi : 10.1002 / andp.18431361110 ( online at Gallica , Bibliothèque nationale de France).
- ^ A b c William B. Jensen: The Grove and Bunsen Cells . Notes from the Oesper Collections (= Notes from the Oesper Collections . No. 23 ). October 29, 2013 (English, online at Digital Collection and Repositories, University of Cincinnati Libraries [PDF; accessed April 1, 2019]).
- ↑ John T. Stock: Bunsen's Batteries and the Electric Arc . In: American Chemical Society ACS (Ed.): Journal of Chemical Education . tape 72 , no. 2 . ACS Publications, February 1995, ISSN 0021-9584 , pp. 99-102 , doi : 10.1021 / ed072p99 .
- ^ Théodore Achille Louis du Moncel, Robert Routledge: Electric lighting . London 1883, p. 28 (English, online in the Internet Archive): “inodorous by hermetically clsoing”
- ^ Robert Bunsen, Henry Roscoe: Photochemical investigations . Second treatise. Measures of the chemical effects of light. In: Johann Christian Poggendorff (Ed.): Annals of Physics and Chemistry . tape 176 , no. 1 . Johann Ambrosius Barth, Leipzig 1857, p. 43–88 , doi : 10.1002 / andp.18571760104 ( online at Gallica , Bibliothèque nationale de France).
- ↑ Robert Bunsen: Representation of the lithium . In: Friedrich Wöhler, Justus Liebig, Hermann Kopp (Hrsg.): Annalen der Chemie und Pharmacie . tape 94 , no. 1 . CF Winter, Leipzig and Heidelberg 1855, p. 107–111 , doi : 10.1002 / jlac.18550940112 ( online in the Internet Archive).
- ↑ Robert Bunsen: Note on the electrolytic extraction of earth and alkali metals . In: Johann Christian Poggendorff (Ed.): Annals of Physics and Chemistry . tape 168 , no. 8 . Johann Ambrosius Barth, Leipzig 1854, p. 648–651 , doi : 10.1002 / andp.18541680812 ( online in the Internet Archive).
- ↑ Hermann Kolbe : Decomposition of valeric acid by the electric current . In: Friedrich Wöhler, Justus Liebig (Ed.): Annals of Chemistry and Pharmacy . tape 64 , no. 3 . CF. Winter, Heidelberg 1848, p. 339–341 , doi : 10.1002 / jlac.18480640346 ( online at the Bayerische Staatsbibliothek BSB ).
- ↑ Hermann Kolbe: Studies on the electrolysis of organic compounds . In: Friedrich Wöhler, Justus Liebig (Ed.): Annals of Chemistry and Pharmacy . tape 69 , no. 3 . CF Winter, Heidelberg 1849, p. 257–294 , doi : 10.1002 / jlac.18490690302 ( online in the Internet Archive [accessed on July 31, 2016]): "To obtain important information about their chemical constitution through the electrolytic decomposition of organic compounds."
- ^ Henri Moissan: Action d'un courant électrique sur l'acide fluorhydrique anhydre . In: Comptes rendus hebdomadaires des séances de l'Académie des sciences . tape 102 . Gauthier-Villars, Paris January 1886, p. 1543–1544 ( online at Gallica ).
- ^ Théodore Achille Louis du Moncel: L'Eclairage Electrique . Paris 1879, p. 282 (French, online in the Internet Archive (French), online (English translation, p. 289) [accessed April 1, 2019]).
- ^ Massimo Guarnieri: Switching the Light: From Chemical to Electrical [Historical] . In: IEEE Industrial Electronics Magazine . tape 9 , no. 3 , September 2015, ISSN 1932-4529 , p. 44-47 , doi : 10.1109 / MIE.2015.2454038 ( ieee.org ).
- ^ Théodore Achille Louis du Moncel: L'Eclairage Electrique . Paris 1879, p. 28–29 (French, online in the Internet Archive (French), online (English translation, p. 29) [accessed on April 14, 2019]): "in units of electromotive force or in volts from 1,888 to 1,964"