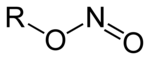Nitrites
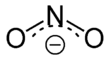
Nitrites are salts (M + NO 2 - , M: monovalent cation ) and esters (R – O – N = O, R: organic residue) of the nitrous acid HNO 2 . The isomeric nitro compounds (R-NO 2 ) in which the organic residue is directly bound to the nitrogen are to be distinguished from the esters of nitrous acid .
Examples
|
|
||||||||||||||||||||||||||||
structure
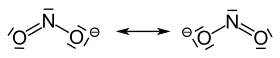
The nitrite ion has an angled structure with a bond angle of 115 °. The figure shows the two mesomeric boundary structures :
Occurrence
Nitrite ions are formed by chemical reaction of nitrous gases with oxygen and moisture in the air (see acid rain ) as well as in the soil in bodies of water and in sewage treatment plants by nitrite bacteria ( nitrosomonas ) by oxidation from ammonium ions with consumption of oxygen. In the process of protein breakdown, they are the intermediate product in the complete oxidation of ammonia to nitrate ( nitrification ). They also arise under anaerobic conditions through bacterial reduction from nitrate ions ( nitrate reductase ).
A high content of nitrites in the water indicates that the water is heavily polluted with nitrogen compounds.
In industry, the treatment of metal surfaces, galvanic processes and the cleaning of exhaust gases containing nitrogen oxides can produce toxic nitrite wastewater that must be treated before disposal.
use
Nitrites in the form of potassium (E 249) and sodium nitrite (E 250) may be used as food additives as color stabilizers in nitrite curing salt . However, nitrite is also used illegally e.g. B. used for dyeing tuna .
The use of nitrites is mandatory in sausage production, as it prevents the development of the highly dangerous botulism bacterium Clostridium botulinum . There is no such requirement in Switzerland. Air-dried meat products such as Parma ham or chorizo do not require nitrite.
At higher temperatures, nitrosamines can be formed together with protein components of the food , which are considered carcinogenic (carcinogenic). Therefore, cured meat products should never be grilled.
Toxicity
Nitrites are toxic . Nitrite ion reacts with the iron atoms in iron-containing enzymes in cellular respiration and in hemoglobin . The latter is oxidized to methaemoglobin by nitrite (see also methaemoglobinaemia ) , whereby the ability to transport oxygen is lost. Nitrites are also involved in the formation of carcinogenic nitrosamines .
Nitrite is also toxic to fish and other aquatic animals at concentrations above 0.1 mg / l, although there is a strong dependency on the pH value of the water, as only nitrous acid can get into the body through the gills . The LD 50 (acute toxicity) for nitrous acid is around 0.01 mg / l for all freshwater fish species. Nitrite ions find another way into the fish through an active transport mechanism, which actually serves to absorb chloride ions when their concentration in the water is well below approx. 15 mg / l. Nitrite is far less toxic in seawater. Due to the active transport mechanism, the uptake of chloride ions, this active uptake is in competition with the nitrite uptake. With a chloride concentration of 20 g / l and a concentration of 1 mg / l nitrite, there are 25,000 chloride ions for each nitrite ion. These displace any nitrite, so that nitrite is absorbed to a significantly lower extent.
Organic nitrites act in the body as nitrogen monoxide donors and thus have its effect, which use a second messenger mechanism to relax the smooth muscles and cause vasodilation . In the event of an overdose of nitrites that are also used therapeutically , there can be a sharp drop in blood pressure , circulatory collapse and even shock . Nitrites such as isobutyl nitrite and amyl nitrite are inhaled as aphrodisiacs under the name Poppers .
Treatment of wastewater containing nitrite
Various methods are possible for the removal of nitrites from sewage before they are discharged into a receiving water or sewer system. The main procedures are as follows:
- Treatment of waste water with oxidative chemicals. This are hypochlorites and peroxides suitable. The pH value is important in the implementation. In weakly acidic conditions, the reaction rate is high and is quantitative according to the following equations:
or
In the basic range, however, the reactions proceed very slowly, while in the strongly acidic range at pH <2.0 nitrous gases are formed. The equation for the latter reaction is as follows:
The disadvantage of using hypochlorite for treatment is that the water is salted up with chlorides and nitrates. Furthermore, organic halogen compounds ( AOX ) of hydrocarbons (HC) can arise if the water contains HC. If peroxides are used, there is a risk of catalytic decomposition by metals or metal salts. This can cause uneconomical consumption of peroxide.
- Another way to remove nitrites is to break them down with amidosulfonic acid or urea . The degradation, which leads to the reduction of nitrites, takes place for the sulfonic acid according to the equation
and accordingly for urea:
When using sulfamic acid , the formation of sulfates and free acids must be taken into account. The latter requires neutralization, so that the content of salts in the treated wastewater is increased. Furthermore, nitrogen oxides can be formed in unfavorable conditions. These disadvantages do not occur when using urea if nitrous gases are prevented from outgassing. This can be achieved if the degradation is carried out in a closed reactor. At the same time, ammonium (NH 4 + ) can also be broken down using the following equation:
The use of urea is ecologically beneficial, as only the gases nitrogen and carbon dioxide and water are produced as end products . An accumulation of salt in the wastewater is avoided.
proof
Nitrite can be detected by means of a color reaction. To do this, the aqueous sample is mixed with a little Lunge's reagent (acid, partially stable solution of sulfanilic acid and α-naphthylamine ) according to the reaction of Johann Peter Grieß from 1858. Red-violet coloration due to the azo dye formed indicates nitrite.
This reaction is further developed by photometric measurement for the quantitative determination of nitrite z. B. used in water. It is standardized in DIN EN 26 777 (until 1993: DIN 38 405 D10).
See also
Web links
Individual evidence
- ↑ Dietmar Kunath: Nitrites. In: In: Claus Schaefer, Torsten Schröer (Hrsg.): The large lexicon of aquaristics. Eugen Ulmer, Stuttgart 2004, ISBN 3-8001-7497-9 , p. 708.
- ^ Gerhard Gutekunst, Waldemar Mzyk. In: WLB water, air and soil. 1-2, 1991, p. 39.
- ↑ Health Department of the Canton of Basel-Stadt: Illegal coloring of tuna meat. In: gd.bs.ch . May 28, 2019. Retrieved May 28, 2019 .
- ↑ Dietmar Kunath: Nitrites. 2004.
- ↑ Armin Glaser: Advisor seawater chemistry. 2008, Rüdiger Latka Verlag, ISBN 978-3-9810570-2-7 .
- ^ Gerhard Gutekunst, Waldemar Mzyk. In: WLB water, air and soil. 1-2, 1991, pp. 39-40.
- ^ Gerhard Gutekunst, Waldemar Mzyk. In: WLB water, air and soil. 1-2, 1991, p. 40.