Bond angle
In chemistry and molecular physics, the bond angle is the angle between covalent bonds of an atom to two neighboring atoms. It essentially depends on the atomic orbitals involved in the bonds, but can be influenced to a certain extent by steric interactions . The bond angles and thus the geometric structures of a molecule can be explained with the help of the VSEPR model . For compounds that contain elements from the subgroups, however, the VSEPR model usually fails.
For the bond angles between atoms in molecules whose orbitals are hybridized, there are specific theoretical angles (pseudostructure):
- sp 3 - hybrid orbitals in molecules align with one another at a tetrahedral angle of 109.5 °.
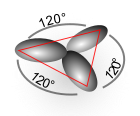
- sp 2 hybrid orbitals are planar-trigonal to one another and form an angle of 120 °.
- sp hybrid orbitals align themselves linearly, resulting in a bond angle of 180 °.
Pseudo structure at
sp 3 - hybrid orbitals
The actual bond angles (real structure) in many molecules that have a tetrahedral, trigonal or linear structure, however, differ to different degrees from the angles mentioned above (pseudo structure). The actual bond angle in the water molecule is not 109.5 °, but 104.45 °, since the non-bonding electron pairs slightly repel the bonding ones. The ammonia molecule also has an angle that deviates from 107 °. The deviation is smaller than in the water molecule, since ammonia only has one free electron pair.
One method for measuring bond angles is NMR spectroscopy . The dependence of the coupling constant and bond angle between two CH bonds that occurs in NMR is called the Karplus relationship after its discoverer Martin Karplus . Molecular vibrations usually involve a periodic deformation of bond angles.
See also
Individual evidence
- ↑ a b c d Theodore L. Brown, Bruce Edward Bursten, Harold Eugene LeMay: Chemistry study compact . Pearson Deutschland GmbH, 2011, ISBN 978-3-86894-122-7 , pp. 343 ( limited preview in Google Book search).
- ↑ Richard E. Dickerson: Principles of Chemistry . Walter de Gruyter, 1988, ISBN 978-3-11-009969-0 , p. 488 ( limited preview in Google Book search).
- ↑ Erwin Riedel, Christoph Janiak: Inorganic Chemistry . Walter de Gruyter GmbH & Co KG, 2015, ISBN 978-3-11-035528-4 , p. 106 ( limited preview in Google Book search).
- ^ Thomas Hill: Examination Knowledge Physikum . Georg Thieme Verlag, 2009, ISBN 3-13-152131-7 , p. 399 ( limited preview in Google Book search).
- ↑ Arthur Beiser: Atoms, Molecules, Solids . Springer-Verlag, 2013, ISBN 978-3-322-91105-6 , pp. 134 ( limited preview in Google Book search).
- ↑ Paula Yurkanis Bruice: Organic Chemistry compact studying . Pearson Deutschland GmbH, 2011, ISBN 978-3-86894-102-9 , pp. 491, 551 ( limited preview in Google Book search).