urea
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
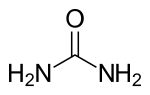
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | urea | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | CH 4 N 2 O | |||||||||||||||||||||
| Brief description |
colorless and odorless, crystalline solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 60.06 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.34 g cm −3 |
|||||||||||||||||||||
| Melting point |
133 ° C (decomposition) |
|||||||||||||||||||||
| Vapor pressure |
1.6 · 10 −3 Pa (25 ° C) |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
very good in water:
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−333.1 kJ mol −1 |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Urea ( Latin and English urea , not to be confused with uric acid ), chemically the diamide of carbonic acid , is an organic compound . It plays an important role in many biological processes such as the metabolism of proteins . In mammals , urea is a metabolic product that is subject to urine and is excreted mainly with the urine and to a lesser extent with the sweat .
Pure urea is a white, crystalline, odorless, non-toxic and hygienically harmless solid that dissolves well in water. With a production volume of around 200 million tons per year, it is one of the most widely produced chemicals. Urea is a widely used nitrogen fertilizer and raw material for the chemical industry , for example for the production of urea resins , which are used as adhesives , for impregnation or insulation . Urea also serves as the basis for the synthesis of melamine , barbituric acid , caffeine , hydrazine and other chemicals.
In the diet of ruminants , urea can be used as a nitrogen source for the formation of proteins. The list of indispensable drugs of the World Health Organization includes urea as a keratolytic and skin moisturizing agent used in ointments and creams for dry, cracked and itchy skin conditions.
The production of urea from inorganic starting materials was an important conceptual milestone in chemistry and is considered to be the birth of biochemistry . This synthesis showed that a metabolic product can be made in the laboratory without biological starting materials, and ultimately led to the fall of the doctrine of vitalism .
history

The Dutch chemist Herman Boerhaave discovered urea while examining urine in 1727, which he called sal nativus urinæ , the natural salt of urine. Hilaire-Marin Rouelle also succeeded in obtaining urea in 1773 using an alcoholic extract from urine residues. In 1797, William Cruickshank showed that urine contained a crystallizable substance that could be precipitated by nitric acid. With that he had represented the urea. Louis-Nicolas Vauquelin (with his doctoral supervisor A. F. de Fourcroy ) detected urea in animal urine and reported about it in 1800. He also proved that the substance found by Rouelle was identical to a substance that Carl Wilhelm Scheele had obtained by treating urine with concentrated nitric acid , and named the substance urea. In 1812 the zoologist John Davy , without knowing what the reaction product was, made artificial urea from phosgene and ammonia. William Prout determined the chemical composition of urea in 1817.
Friedrich Wöhler first produced urea in 1828 through the reactions of silver cyanate (AgOCN) and ammonium chloride (NH 4 Cl)
or from lead cyanate (Pb (OCN) 2 ) and liquid ammonia .
He recognized the intermediate compound ammonium cyanate (NH 4 OCN) as the actual urea source.
Urea is considered to be the first organic compound to be synthesized from inorganic raw materials. This contradicted the notion that was widespread at the time that organic substances could basically only be produced by living beings using the so-called vis vitalis (life force). Strictly speaking, Wöhler provided evidence as early as 1824 through the hydrolysis of dicyan to oxalic acid that “life force” is not required for the synthesis of organic molecules.
In 1868 Alexander Basaroff first described the commercially applicable production of urea from ammonium carbamate , which is accessible under pressure from ammonia and carbon dioxide.
Carl Bosch succeeded in implementing this process on an industrial scale in 1922 after ammonia was available in large quantities through the Haber-Bosch process and the required high-pressure technology. With the worldwide construction of Haber-Bosch plants after the Second World War , the production of urea increased rapidly parallel to the production of ammonia.
Occurrence

Urea of a synthetic or biological nature that gets into the environment is usually quickly converted by bacteria into ammonium , nitrite and nitrate ions and thus becomes part of the nitrogen cycle . Together with uric acid , urea is part of the excretions of birds and bats and occurs in bat guano (chiropterite) and in small amounts in guano .
As a mineral, urea is only stable under arid conditions. Urea was found in 1973 as a natural secondary mineral at Toppin Hill on Lake Rason (Western Australia), associated with ammonium naphthitalite , ammonium phosphates and Weddellite . It has been recognized as a standalone mineral by the International Mineralogical Association (IMA) . According to the systematics of minerals according to Strunz (9th edition) , these are listed as "Diverse organic compounds" under system no. "10.CA.35". The systematics of minerals according to Dana , which is also common in English-speaking countries , lists the mineral under system no. "50.4.6.1".
Manufacturing

Urea can be produced on a laboratory scale by reacting ammonia with phosgene or carbonic acid esters or by hydrolysis of cyanamide . Urea is produced industrially in large quantities, around 184 million tons worldwide in 2012. According to the International Fertilizer Industry Association (IFA), a further increase in production capacity of 41 million tons is expected between 2013 and 2018, of which 5 million tons will be in the United States. The capacity expansion in the USA is due to the expansion of shale gas production .
Large plants are used to produce urea, which first use the Haber-Bosch process to produce ammonia and finally urea from natural gas , air and water. Two thirds of the carbon dioxide previously separated during hydrogen production is bound during the production of urea.
The industrial production of urea in a high pressure process goes back to Carl Bosch and Wilhelm Meiser. In 1922, BASF commissioned the first production plant in which ammonium carbamate (NH 4 CO 2 NH 2 ) was formed from ammonia and carbon dioxide in an exothermic reaction of −117 kJ / mol in a high-pressure reactor at 150 bar :
Ammonium carbamate reacts in an endothermic reaction of +15.5 kJ / mol to form urea and water:
It is an equilibrium reaction. An excess of ammonia is used to optimize the yield. Gases returned to the process should be as free of water as possible, since water shifts the equilibrium to the side of the ammonium carbamate. The overall reaction is exothermic. One of the by-products is isocyanic acid .
The reaction can be suppressed by working in an excess of ammonia. BASF initially used excess ammonia to produce ammonium sulfate and ammonium nitrate . At the end of the 1920s, the process was improved and excess ammonia was returned to the production process. Various total cycle processes developed from this, for example from DuPont , Pechiney or Stamicarbon .
The processes differ in the type of decomposition of ammonium carbamate, the separation and recovery of carbon dioxide and ammonia, and the processing of the urea. The reaction temperatures are between 170 and 220 ° C, depending on the process, the reaction pressure between 125 and 250 bar. All modern industrial processes have in common that the excess gases are fed back into the reactor, whereby stripping processes are used.
To produce one tonne of urea, 0.58 tons of ammonia and 0.76 tons of carbon dioxide are required. Depending on the process, between 85 and 160 kWh of electrical energy and between 0.9 and 2.3 tons of steam are required.
An important consideration in the process design is the limitation of the content of biuret , which is formed from urea when exposed to temperature and which is present as an impurity in industrially produced urea. The content of biuret in urea must be limited, usually less than 1.0%, since biuret has an inhibiting effect on the development of seeds and healthy plant growth.
The urea, which initially occurs in solution, is traditionally converted into a fine-grain bulk material with a grain size of around two millimeters by prilling, also known as spray crystallization, and sold in bags or loose. For this purpose, a liquid urea melt is divided into small drops in a prilling tower and allowed to free fall; Large fans at the top of the tower suck in cold fresh air in countercurrent, causing the liquid to solidify into solid spheres as it falls. As a result of the melting and cooling, a relatively small surface is formed, the urea becomes less hygroscopic and the flow properties are maintained over several months. Since prills have disadvantages, particularly in terms of size and strength, solid urea is almost exclusively granulated in newer fertilizer plants, with spray granulation in a fluidized bed being the most modern technology and almost exclusively used in new plants. The largest plants in the world produce around 4,000 tons of urea per day in one production line.
Urea with a lower biuret content can be obtained from the mother liquor by crystallization . The biuret remains in the aqueous phase and can thus be separated. Further processing can also be done by prilling.
properties
Physical Properties
Urea is a colorless crystalline solid with a density of 1.32 g / cm 3 under standard conditions . It melts in the range from 132.5 to 134.5 ° C with decomposition. The vapor pressure at 75 ° C is 0.2 Pascal . Urea dissolves very well in water and other polar solvents; one gram of urea dissolves in 1.5 ml of water, 10 ml of ethanol, 6 ml of methanol or 2 ml of glycerine.
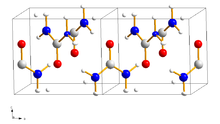
Urea crystallizes in the tetragonal crystal system with the space group P 4 2 1 m (space group no. 113) with the lattice parameters a = 564.6 pm, c = 470 pm and a ratio of a : c = 1: 0.833. It develops colorless to pale yellow or pale brown, needle-shaped crystals. Solid urea has two N – H – O hydrogen bonds on the oxygen atom; the distance between the oxygen and hydrogen atoms is 299 picometers and 304 picometers. The existing hydrogen bonds break under high pressure and new bonds are formed. From a pressure of 4800 bar an orthorhombic phase forms with the space group P 2 1 2 1 2 1 (space group no. 19) .
The crystal structure consists of ribbons in the shape of a helix, which can store organic compounds as guest molecules. In these clathrates , the organic guest molecules are held in the channels that are formed by interpenetrating helices of hydrogen-bonded urea molecules. This behavior can be used to separate linear and branched hydrocarbon mixtures, for example in urea extractive crystallization .
Chemical properties
- Urea dissolves easily in water and ethanol , but not in diethyl ether or benzene . The aqueous solution reacts neutrally. The protonated form [NH 2 C (OH) NH 2 ] + is formed with acids . The urea salts of nitric acid are explosives . When the aqueous solution is heated with acids, urea breaks down into carbon dioxide and ammonium salts , when heated with alkalis it breaks down into carbonates and ammonia.
- When heated above the melting point, urea first condenses to form isocyanic acid , splitting off ammonia , which then reacts with urea to form biuret :
At higher temperatures, other condensation products such as triuret , guanidine and melamine are formed .
These transamidations are important reactions for the general representation of urea derivatives:
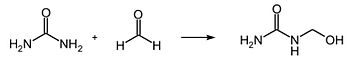
- Urea reacts with formaldehyde in an addition reaction to form methylolureas , which have hydroxymethyl groups:
These react in a condensation reaction with the release of water and the formation of methylene groups to form urea resins :
- Urea forms a hydrogen peroxide-urea adduct with hydrogen peroxide, the carbamide peroxide . This is a water-soluble crystalline adduct that forms when urea is recrystallized with concentrated hydrogen peroxide solution. It contains around 35% hydrogen peroxide.
The hydrogen can be partially or completely replaced by other substituents through various reactions. With benzoyl chlorides, for example, imides such as benzoylureas are formed , and sulfonylureas are formed through reaction with sulfonyl chloride .
By heating to about 200 to 300 ° C to form cyanuric acid .
- By introducing chlorine into a 20% urea solution and then adding 20% sodium hydroxide solution , hydrazine is produced in a 50% yield in a Hofmann rearrangement .
- Urea breaks down to ammonia and carbon dioxide at very high temperatures
The resulting ammonia is in the SNR method used to the information contained in exhaust gases of power plants and combustion engines, nitrogen oxides to elemental nitrogen to reduce
Molecular Properties
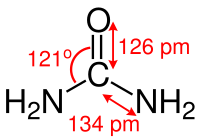
The carbon atom of the urea molecule is coordinated in a trigonal planar manner (roughly: sp 2 -hybridized ), the carbon-nitrogen bonds have a significant double bond character and, compared to the carbon-nitrogen bonds in alkylamines , are about 10 picometers shorter. The atomic distance between nitrogen and hydrogen is 105 picometers, the distance between nitrogen and carbon is 134 picometers, and the distance between carbon and oxygen is 126 picometers. The N – C – O angle is 121 °. The molecule has overall C 2v symmetry .
The stretching vibration ν N – H is in the infrared range of the spectrum at a wave number of 3396 cm −1 and thus higher than that of ammonia, which has a wave number of 3372 cm −1 . The ν C = O stretching vibration is 1687 cm −1 and indicates a resonance between a carbonyl and alcoholate structure. The ν C-N stretching vibration is 1465 cm −1 .
use
An industrial use of urea is the production of melamine , which is e.g. B. is processed with formaldehyde to synthetic resins, and urea-formaldehyde resins ( urea resin , so-called UF resins ), the z. B. be used for the production of chipboard . Otherwise, urea is mainly used as a nitrogen fertilizer or as a reducing agent for nitrogen oxides in the SNCR process. The demand for urea increased steadily over the years, between 1960 and 1970 alone the demand and production capacity tripled. Between 1990 and 2010, demand grew steadily by a little more than 3% annually, with the installed capacity exceeding demand by around 10 to 20%. The drivers for a further increase in demand could be both increasing requirements for nitrogen oxide reduction in road traffic and the expansion of biofuel capacity .
Nitrogen fertilizer

At 46.63%, urea has the highest nitrogen content of all conventional nitrogen fertilizers; ammonium nitrate, which is also often used, has a nitrogen content of 35%. Many soil bacteria have the enzyme urease , which catalyzes the conversion of urea into ammonia or ammonium ions and hydrogen carbonate ions .
In order not to be lost as a gas in the atmosphere, ammonia must be fixed to the ammonium ion with water or an acid:
Ammonia-oxidizing nitrite bacteria as Nitrosomonas oxidize this with production of energy in the so-called nitrification to nitrite :
Nitrite oxidizers such as Nitrobacter further oxidize the resulting nitrite to nitrate :
Plants easily absorb ammonium ions and nitrate; they are the predominant sources of nitrogen for plant growth.
Urea is the most widely used nitrogen fertilizer worldwide, calculated on the basis of the nitrogen content. In various regions, such as Asia, the percentage in 1997 was over 50%.
|
Share of various nitrogen fertilizers in global consumption (based on nitrogen; 1997) |
|
|---|---|
| fertilizer | Percentage |
| urea | 44.22 |
| Ammonium nitrate | 9.52 |
| ammonia | 5.67 |
| Ammonium nitrate urea solution | 4.96 |
| Calcium ammonium nitrate | 4.31 |
| Ammonium sulfate | 3.06 |
| Other | 28.26 |
Consumption rose particularly strongly in Asian countries. In 2013, India, China and Pakistan were the world's largest consumers of urea.
| List of the ten countries with the highest urea consumption | ||
|---|---|---|
| country | year |
Consumption in millions of tons |
| India | 2013 | 30.60 |
| China | 2012 | 28.50 |
| Pakistan | 2013 | 5.89 |
| United States | 2013 | 5.60 |
| Indonesia | 2013 | 4.77 |
| Brazil | 2013 | 4.56 |
| Canada | 2013 | 3.38 |
| Thailand | 2013 | 2.37 |
| Egypt | 2013 | 1.93 |
| Iran | 2013 | 1.84 |
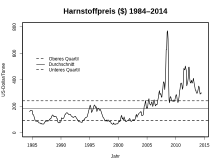
The urea price hovered around US $ 100 / ton for a long time , but rose rapidly from 2003 to its peak in August 2008 at $ 770 / t. It then fell again and was just under $ 200 / t in June 2016.
Medical applications
In pharmacy , urea is used as a keratolytic (i.e., as an active ingredient that dissolves horns ). This property is used in various recipes. For example, it is highly concentrated (40%) in pastes together with an antifungal agent ( antimycotic ) against nail fungus (onychomycosis), whereby the urea softens the nail so that the infected nail substance can be removed piece by piece. It is also used as a moisturizer in ointments to combat atopic eczema and lichen diseases . In the past, urea in aqueous solution was prescribed as a diuretic for pleurisy and cirrhosis of the liver .
Urea is used in first aid as a component of cold packs to cool strains or bruises. These consist of two separate areas, one of which is urea and the other is water. If the separation is broken, the urea dissolves in the water. Since the lattice energy is greater than the hydration energy, the dissolving process draws energy from the environment and cools it down.
The administration of 13 C-labeled urea and the subsequent detection of 13 C-labeled carbon dioxide using a 13 C urea breath test enables the detection of Helicobacter pylori in the stomach. The released carbon dioxide indicates the presence of the enzyme urease, which the bacterium uses to produce ammonia from urea and thus increase the pH of the stomach environment. The bacterium is one of the causes of ulcers .
Some derivatives of urea are active ingredients in pharmaceuticals and pesticides. Barbituric acid and barbiturates are derivatives of urea, which can be obtained from diethyl malonate and its derivatives and urea.
Sulphonylureas are used as oral antidiabetic agents , and they also form an important group of herbicides .
The urea concentration in the blood or the blood urea nitrogen linked to it by a factor of 0.467 are among the kidney retention parameters that are determined as medical indicators in nephrology for assessing the performance of the kidneys . Elevated values can indicate impaired kidney function, but are influenced in many ways, for example by protein intake.
The urea reduction ratio (urea reduction ratio (URR)) is a measure of the elimination of solutes during hemodialysis . The urea reduction ratio is the fraction of the blood urea concentration that is removed in relation to the total blood urea concentration during hemodialysis treatment.
environmental Protection
Urea is used to reduce nitrogen oxides in the exhaust gas from power plants and internal combustion engines. The SNCR process ( selective non-catalytic reduction ) is used in power plants , primarily in smaller systems . In the so-called SCR process ( selective catalytic reduction ), which is used in power plants and increasingly in vehicle technology, urea or ammonia is injected into the hot exhaust gas flow. The urea decomposes into ammonia, which reduces the nitrogen oxides in a downstream catalytic converter . In automotive engineering, an aqueous solution with 32.5% urea is used, which is standardized under the designation AUS 32 . The consumption of urea solution is about 2 to 8% of the fuel consumption.
Food production
Urea is added to food as a stabilizer . In the EU it is only approved as a food additive with the designation E 927b for chewing gum without added sugar. It acts as an acid regulator in the mouth by splitting off ammonia.
Urea plays a role as a nitrogen source in cattle farming . Urea provides the nitrogen for nutrition, but the cow also needs energy and minerals in the rumen to produce proteins. Theoretically, 100 grams of urea produce 2875 grams of crude protein. Since 2008, urea may only be used for supplementary feeding if the livestock owner meets certain requirements according to the feed hygiene ordinance.
The addition of urea in higher concentrations to aqueous solutions leads to a denaturation of proteins ; urea therefore acts as a denaturant or as a chaotropic compound . However, low concentrations of urea can have the opposite effect, such as increasing the hydrophobic effect and thus stabilizing the protein structure.
The urea content of rays and sharks means that they first have to be fermented for several weeks before they can be consumed in order to break down the urea into ammonia and release it into gas. Fermented skate and Greenland sharks , under the name Gammel rays and Hákarl known deemed Icelandic specialties.
Other uses
Urea is used as a substitute for road salt , but because of its higher price this is only done in special cases, for example for de-icing movement areas at airports . In Switzerland, use is only permitted at airports.
Urea is in the urea extraction crystallization , a process for separating linear paraffins from hydrocarbon mixtures through the formation of urea n paraffin - clathrates used. The purpose of the separation is to lower the pour point of mineral oil products; high-purity n- paraffins are obtained as by-products . The method can be used to separate fatty acids and fatty alcohols .
The denaturing effect of urea on proteins is used in urea-PAGE , the urea- polyacrylamide gel electrophoresis . The urea concentrations used are in the order of 4 to 8 molar . In contrast to SDS-PAGE , Urea-PAGE practically does not change the charge of the proteins, which enables the separation of proteins with the same molecular mass but different charges. The effective volume of the individual protein molecules is greater with urea-PAGE than with native-PAGE , and the aggregates of the protein molecules break down into their subunits. If the protein molecules or their aggregates are stabilized by disulfide bridges , reducing thiols are added to urea-PAGE , similar to SDS-PAGE.
Urea is often used as a moisture factor in cosmetics because of its high water-binding capacity , usually declared as urea .
A fire extinguishing powder based on potassium hydrogen carbonate uses urea as a component. The powder is used to fight BCE fire class fires . Use is not permitted in Germany.
Benzoylureas are insecticides that act as a chitin inhibitor .
In the direct urea fuel cell (DUFC), urea serves as a hydrogen supplier. In addition to generating energy, the process is also suitable for removing urea from wastewater.
Biological importance
Many vertebrates , such as squid like sharks and rays , amphibians and mammals, produce urea as an end product of the metabolism of nitrogen compounds such as amino acids . He is one of the urinary substances . The breakdown of amino acids initially produces ammonia, which is toxic to cells in high concentrations .
The formation of urea takes place in the liver through the reaction of two molecules of ammonia with one molecule of carbon dioxide in the urea cycle . It is transported from the liver to the kidney and excreted in the urine. About half of the solids content in urine consists of urea. Urea cycle defects are hereditary metabolic diseases that are associated with a disruption of ammonia conversion. They lead to an increased level of ammonia in the blood, which damages nerve cells. Small amounts of urea are excreted in humans through sweat and intestinal secretions. The human body produces around 20 to 30 grams of urea per day. Various living things use urea as a biogenic antifreeze agent . Sharks and rays do not excrete all urea, but use it to regulate osmosis .
A high protein intake leads to increased urea levels even with normal kidney function, which makes it a poor kidney parameter. Diseases such as acute or chronic kidney failure , as well as diabetic impaired kidney function, can lead to increased urea levels in serum / plasma (normal value: 10–50 mg / dl). In the case of (pre-) terminal renal failure , the urea concentration in the serum is better suited to assess the severity of the uremia than the serum creatinine concentration.
The milk urea content is the content of urea in milligrams per liter of milk and is an important benchmark for the optimal protein and energy supply of the cow. The urea content of the milk is determined by the feed with crude protein per animal and day, the content of flow protein and the carbohydrates fermentable in the rumen and serves as a measure for the utilization of the raw feed protein. A relative over- or undersupply of crude protein indicates management errors with threats to animal health.
toxicology
Urea is to be regarded as practically non-toxic. No toxicity was found in feeding experiments with rats at a dose of 20 grams per kilogram of body weight or when piglets were fed up to 4 grams per kilogram of body weight for several days. No influence of urea administration on the development of fetuses in rats and mice could be determined.
Oral administration of high-dose urea solutions to dogs for several days caused weakness, loss of appetite, vomiting and gagging, diarrhea and decreased body temperature, which led to a coma. In tests with nude mice to which pure urea was applied to the skin, no changes in the skin could be found. However, urea increases the skin penetration of other substances.
Verification procedure
Precipitation as urea nitrate is suitable for qualitative proof . In acetic acid solution, urea can be converted into dixanthylurea with xanthydrol and precipitated. Urea can be enzymatically split into carbon dioxide and ammonia by means of urease . Food analysis uses this cleavage for the quantitative detection of ammonia by means of the blue-colored indophenol ion in the Berthelot reaction .
literature
- Dieter Fromm, Dietrich Lützow: Modern processes in large-scale chemistry: urea. In: Chemistry in Our Time . 13th year No. 3, 1979, pp. 78-81, doi: 10.1002 / ciuz.19790130303 .
- Jozef Meessen: Urea. In: Ullmann's Encyclopedia of Industrial Chemistry . Vol. 37, Wiley-VCH Verlag, Weinheim 2012, pp. 657-695, doi : 10.1002 / 14356007.a27_333.pub2 .
- Jozef Meessen: Urea synthesis. In: Chemical Engineer Technology . 86th year No. 12, 2014, pp. 2180–2189, doi: 10.1002 / cite.201400064 . (Review article on history, thermodynamics and current manufacturing processes).
Web links
Individual evidence
- ↑ Entry on UREA in the CosIng database of the EU Commission, accessed on December 28, 2019.
- ↑ Entry on E 927b: Carbamides in the European database on food additives, accessed on June 27, 2020.
- ↑ a b Entry on urea. In: Römpp Online . Georg Thieme Verlag, accessed on May 26, 2014.
- ↑ a b c d e f Entry on urea in the GESTIS substance database of the IFA , accessed on December 19, 2019(JavaScript required) .
- ↑ Walter Klöpffer: behavior and degradation of environmental chemicals Physico-chemical bases . John Wiley & Sons, 2013, ISBN 3-527-67212-5 , pp. 574 ( limited preview in Google Book search).
- ↑ Bordwell pK a -Table
- ↑ pKa Data , Compiled by R. Williams (PDF, 78 kB).
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Analytical Chemistry, pp. 8-120.
- ↑ Entry on urea in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on October 17, 2016.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-20.
- ↑ Entry on sweat. In: Römpp Online . Georg Thieme Verlag, accessed on November 5, 2019.
- ↑ Peter Shaw, Ephraim Chambers: A New Method of Chemistry. Volume 2, J. Osborn and T. Longman, London 1727, ( p. 193: Process LXXXVII. Online ).
- ^ Louis Rosenfeld: William Prout: Early 19th Century Physician-Chemist. In: Clinical Chemistry. 49, 2003, pp. 699-705, doi: 10.1373 / 49.4.699 .
- ↑ The results of the trials of various acids, and some other substances, in the treatment of the Lues Veneres etc. by William Cruickshank . In: John Rollo († 1809): An account of two cases of the diabetes mellitus… London 1797, Part II, pp. 141–225 ( digitized version ).
- ^ William Coulson: On the diseases of the bladder and prostate gland . John Churchill, London 1857, p. 15 ( digitized version ).
- ↑ a b Peter Dilg: Urea synthesis. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 535.
- ^ William Prout: Observations on the Nature of some of the proximate Principles of the Urine; with a few remarks upon the means of preventing those diseases, connected with a morbid state of that fluid. In: Medico-surgical transactions. Volume 8, 1817, pp. 521-544, 596-7-596-9. PMID 20895332 , PMC 2128986 (free full text).
- ↑ Friedrich Wöhler: About artificial formation of urea. In: Annals of Physics and Chemistry. 88, 1828, pp. 253-256, doi: 10.1002 / andp.18280880206 .
- ^ Burchard Franck: 250 years of chemistry in Göttingen. In: Hans-Heinrich Voigt (Ed.): Natural sciences in Göttingen. A series of lectures. ( Göttinger Universitätsschriften . Volume 13). Vandenhoeck + Ruprecht, Göttingen 1988, ISBN 3-525-35843-1 , p. 72. ( limited preview in Google book search)
- ↑ H. Kolbe, Dr. Wilm, Dr. Wischin, Mr. Irelan, Alexander Basaroff, Dr. Theilkuhl: XXV. — Chemical contributions. In: J. Chem. Soc. 21, 1868, pp. 192-197, doi: 10.1039 / JS8682100192 .
- ↑ a b Patent US1429483 : Process of manufacturing urea. Published on September 19, 1922 , inventors: Carl Bosch, Wilhelm Meiser.
- ↑ Entry on guano. In: Römpp Online . Georg Thieme Verlag, accessed on May 22, 2012.
- ↑ Michael Fleischer: New Mineral Names. In: American Mineralogist. 59, 1974, pp. 874-875.
- ^ PJ Bridge: Urea, a new mineral, and neotype phosphammite from Western Australia. In: Mineral. Mag. 39, 1973, pp. 346-348.
- ↑ Ceresana: Urea market study , accessed on May 17, 2013.
- ^ Food and Agriculture Organization of the United Nations: World fertilizer trends and outlook to 2018. Rome 2015, ISBN 978-92-5-108692-6 , p. 16.
- ↑ a b c d e f g James A. Kent (Ed.): Riegel's Handbook of Industrial Chemistry . Van Nostrand Reinhold, 1974, ISBN 0-442-24347-2 , pp. 104-111.
- ↑ a b c d e f g h i j Jozef Meessen: Urea. In: Ullmann's Encyclopedia of Industrial Chemistry . Vol. 37, Wiley-VCH Verlag, Weinheim 2012, pp. 657-695, doi: 10.1002 / 14356007.a27_333.pub2 .
- ↑ a b Jozef Meessen: Urea synthesis. In: Chemical Engineer Technology. 86, 2014, pp. 2180-2189, doi: 10.1002 / cite.201400064 .
- ^ ARC Haas, JN Brusca: Biuret, Toxic Form of Nitrogen. In: California Agriculture. 8, 1954, p. 11.
- ↑ Rudolf Gaedeke, Friedrich Wolf, Rita Otto: To influence the structure of urea prills by cooling melt droplets under defined discharge conditions Part 1. In: Hercynia-Ökologie und Umwelt in Mitteleuropa. 20, 2014, pp. 403-410.
- ↑ Patent US3025571 : Process for producing prilled uea of low biuret content. Published on March 20, 1962 , inventors: Jack E. Beecher, Robert J. Kallal u. a.
- ^ Remington: The Science and Practice of Pharmacy. Lippincott Williams & Wilkins Verlag, 2006, ISBN 0-7817-4673-6 , p. 1424.
- ↑ a b c d P. Vaughan, J. Donohue: The structure of urea. Interatomic distances and resonance in urea and related compounds. In: Acta Crystallographica. 5, 1952, pp. 530-535, doi: 10.1107 / S0365110X52001477 .
- ↑ a b mindat: Urea: Urea mineral information and data . Retrieved May 13, 2013.
- ↑ Anna Olejniczak, Kinga Ostrowska, Andrzej Katrusiak: H-Bond Breaking in High-Pressure Urea. In: The Journal of Physical Chemistry C. 113, 2009, pp. 15761-15767 doi: 10.1021 / jp904942c .
- ↑ Kenneth DM Harris: Fundamental and Applied Aspects of Urea and Thiourea Inclusion Compounds. In: Supramolecular Chemistry. 19, 2007, pp. 47-72, doi: 10.1080 / 10610270600977706 .
- ↑ MJ Janssen: The structure of protonated amides and ureas and their thio analogues. In: Spectrochimica Acta. 17, 1961, p. 475, doi: 10.1016 / 0371-1951 (61) 80102-1 .
- ↑ Christian Nitschke, Günter Scherr: Urea Derivatives. In: Ullmann's Encyclopedia of Industrial Chemistry . Vol. 38, Wiley-VCH Verlag, Weinheim 2010, pp. 1-12, doi: 10.1002 / 14356007.o27_o04 .
- ↑ H. Heaney, F. Cardona, A. Goti, AL Frederick: Hydrogen Peroxide-Urea . e-EROS Encyclopedia of Reagents for Organic Synthesis, 2013, doi: 10.1002 / 047084289X.rh047.pub3 .
- ^ Klaus Huthmacher, Dieter Most: Cyanuric Acid and Cyanuric Chloride. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2005. doi : 10.1002 / 14356007.a08_191
- ↑ Patent DE578486 : Production of hydrazine. Published on October 18, 1933 , inventors: Egon Ihwe, Otto Seuffert.
- ↑ Mansel Davies, Leslie Hopkins: The bonding in urea and in the urea ion. In: Transactions of the Faraday Society. 53, 1957, pp. 1563-1569, doi: 10.1039 / TF9575301563 .
- ^ Z. Piasek, T. Urbanski: The infra-red absorption spectrum and structure of urea. In: Bull. Pol. Acad. Sci-tech. X, 1962, pp. 113-120.
- ↑ Bernd Tieke: Macromolecular Chemistry: An Introduction . Verlag Wiley-VCH, 2014, ISBN 978-3-527-33216-8 , pp. 42-45.
- ↑ a b c Description of different technologies and their development potentials for reducing nitrogen oxides in waste gas from waste incineration plants and substitute fuel power plants with regard to performance, costs and energy consumption , brochure of the Federal Environment Agency, 71. 2011 (online)
- ↑ Samuel L. Tisdale, Werner L. Nelson, James D. Beaton: Soil fertility and fertilizers. Macmillian, 1985, ISBN 0-02-420830-2 , pp. 161-168.
- ^ KL Marsh, GK Sims, RL Mulvaney: Availability of urea to autotrophic ammonia-oxidizing bacteria as related to the fate of 14C- and 15N-labeled urea added to soil. In: Biology and Fertility of Soils. 42, 2005, p. 137, doi: 10.1007 / s00374-005-0004-2 .
- ↑ Georg Fuchs: General Microbiology. George Thieme Verlag, 2014, ISBN 978-3-13-444609-8 , p. 40.
- ↑ a b U.S. International Trade Commission: Ammonium Nitrate: A Comparative Analysis of Factors Affecting Global Trade . Investigation No. 332-393, October 1998, p. 2.
- ↑ a b Urea monthly price - US dollars per ton. IndexMundi, accessed July 8, 2016 .
- ↑ Urea, consumption (tonnes) - for all countries , (online)
- ↑ Hellmut Ruck: Handbook for medical foot care . Karl F. Haug Verlag, 2012, ISBN 978-3-8304-7569-9 , p. 121.
- ↑ G. Klemperer, E. Rost: Handbook of the general and special drug regulation theory for doctors . Springer Verlag, 1929, p. 710. (Reprint: ISBN 978-3-642-52556-8 )
- ↑ Robert T. Sataloff: Sataloffs Comprehensive Textbook of Otolaryngology. Jaypee Brothers, 2016, ISBN 978-93-5152745-9 , p. 412.
- ^ Wolfgang F. Caspary, Manfred Kist, Jürgen Stein: Infectiology of the gastrointestinal tract . Springer Medizin Verlag, 2006, ISBN 3-540-41359-6 , p. 166.
- ^ Adolf Baeyer: Studies on the uric acid group. In: Annals of Chemistry and Pharmacy. 131, 1864, pp. 291-302, doi: 10.1002 / jlac.18641310306 .
- ↑ Hans G. Drobny, Martin Schulte, Harry J. Strek: 25 years of sulfonylurea herbicides - a few grams changed the world of chemical weed control. In: Julius Kühn Archive. Volume 434, 2012, pp. 21-33, doi: 10.5073 / jka.2012.434.002 .
- ^ Walter H. Hörl: Dialysis procedures in clinic and practice: technology and clinic. Thieme Verlag, 2003, ISBN 3-13-497706-0 , p. 17.
- ↑ Lynda Anne Szczech, Edmund G. Lowrie, Zhensheng Li, Nancy L. Lew, J. Michael Lazarus, William F. Owen: Changing hemodialysis thresholds for optimal survival. In: Kidney International. 59, 2001, pp. 738-745, doi: 10.1046 / j.1523-1755.2001.059002738.x .
- ^ Robert Bosch GmbH: Kraftfahrtechnisches Taschenbuch. Springer Vieweg, 2014, ISBN 978-3-658-03800-7 , p. 719.
- ↑ Robert Ebermann, Ibrahim Elmadfa: Textbook of food chemistry and nutrition. Springer, 2008, ISBN 978-3-211-48649-8 , p. 663.
- ↑ Katrin Mahlkow-Nerge: Feed urea in milk cattle - careful use is important.
- ↑ M. Holz, M. Mayele: Influence of Additives on Hydrophobic Association in Polynary Aqueous Mixtures. In: DFG Research Report. Thermodynamic Properties of Complex Fluid Mixtures. Wiley-VCH, 2004, ISBN 3-527-27770-6 , pp. 150-183.
- ↑ Ralf Quibeldey: Viking Christmas. In: Spiegel online. December 25, 2004 (online)
- ↑ Chemical Risk Reduction Ordinance , Annex 2.7 , as of January 1, 2016.
- ↑ F. Bengen, W. Schlenk: About novel addition compounds of urea. In: Experientia. 5, 1949, p. 200, doi: 10.1007 / BF02172488 .
- ^ Wilhelm Keim , Arno Behr, Günter Schmitt: Fundamentals of industrial chemistry. Technical products and processes. Otto Salle Verlag, 1985, ISBN 3-7935-5490-2 , p. 250.
- ↑ R. Rigamonti, V. Riccio: Separation of fatty acids and triglycerides with the help of urea addition compounds. In: Fats and Soaps. 54, 1952, p. 193, doi: 10.1002 / lipi.19520540402 .
- ↑ Roger W. Floyd, Marie P. Stone, WK Joklik: Separation of single-stranded ribonucleic acids by acrylamide-agarose-urea gel electrophoresis. In: Analytical Biochemistry. 59, 1974, pp. 599-609, doi: 10.1016 / 0003-2697 (74) 90313-3 .
- ↑ Marina Bährle-Rapp: Springer Lexicon Cosmetics and Body Care . Springer Verlag, 2013, ISBN 978-3-642-24687-6 , p. 255.
- ^ Jürgen Falbe, Manfred Regitz: Römpp Lexikon Chemie . Thieme Verlag, 1997, ISBN 3-13-734710-6 , p. 1330.
- ↑ James E. Wright, Arthur Retnakaran: Chitin and Benzoylphenyl Ureas . Dr W. Junk Publishers, 1987, ISBN 94-010-8638-9 , pp. 101-110.
- ^ Wei Xu, Huimin Zhang, Gang Li, Zucheng Wu: Nickel-cobalt bimetallic anode catalysts for direct urea fuel cell. In: Scientific Reports. 4, 2014, p. 5863, doi: 10.1038 / srep05863 .
- ^ Neil Hazon, Alan Wells et al: Urea based osmoregulation and endocrine control in elasmobranch fish with special reference to euryhalinity. In: Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 136, 2003, pp. 685-700, doi: 10.1016 / S1096-4959 (03) 00280-X .
- ^ Ulrich Kuhlmann: Nephrology: Pathophysiology - Clinic - Renal Replacement Procedure . Georg Thieme Verlag, 2008, ISBN 978-3-13-700205-5 , p. 563.
- ^ R. Oltner, H. Wiktorsson: Urea concentrations in milk and blood as influenced by feeding varying amounts of protein and energy to dairy cows. In: Livestock Production Science. 10, 1983, pp. 457-467, doi: 10.1016 / 0301-6226 (83) 90073-8 .
- ↑ a b Final report of the safety assessment of Urea. In: International journal of toxicology. Volume 24, Suppl 3, 2005, pp. 1-56. PMID 16422263 .
- ↑ Martina Baumgartner, Martina Flöck, Petra Winter: Evaluation of flow injection analysis for determination of urea in sheep's and cow's milk. In: Acta Veterinaria Hungarica. 50, 2002, p. 263, doi: 10.1556 / AVet.50.2002.3.2 .