Carbamide peroxide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
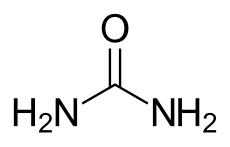
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Carbamide peroxide | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | CH 6 N 2 O 3 | |||||||||||||||||||||
| Brief description |
white crystal powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 94.07 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.390 g cm −3 at 20 ° C |
|||||||||||||||||||||
| Melting point | ||||||||||||||||||||||
| Vapor pressure |
31.1 hPa at 30 ° C |
|||||||||||||||||||||
| solubility | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Carbamide peroxide (urea hydrogen peroxide adduct) is a water-soluble crystalline adduct , in which the recrystallization of urea with concentrated (30 percent) hydrogen peroxide forms solution and contains about 35% hydrogen peroxide. As a solid and anhydrous hydrogen peroxide with higher stability and better controllability, the adduct offers advantages over liquid hydrogen peroxide as an oxidizing agent .
Manufacturing
To produce carbamide peroxide, urea - which is stable to oxidizing agents such as hydrogen peroxide - is dissolved in 30 percent hydrogen peroxide (molar ratio 2: 3) by heating to temperatures below 60 ° C. When cooling, the adduct precipitates in the form of small platelets.
The content determination by titration with potassium permanganate solution gives a hydrogen peroxide content of 35.4% or 97.8% of the theoretical maximum value of 36.2%. The remaining contamination consists of urea.
The adduct can be stabilized by adding approx. 1% sodium pyrophosphate , sodium hexametaphosphate, tartaric acid or EDTA Na 2 , which complex catalytically active heavy metal ions.
properties
Hydrogen peroxide-urea adduct is a readily water-soluble, odorless, crystalline solid that is obtained as a white powder or in colorless needles or small platelets. As a strong oxidizing agent, the compound is oxidizing and can cause skin irritation and serious eye damage.
The anhydrous urea peroxohydrate releases hydrogen peroxide in a controlled manner at room temperature in the presence of catalysts and is therefore suitable as a safe replacement for the unstable aqueous solution of hydrogen peroxide. Because of the tendency to thermal decomposition, which accelerates at temperatures above 82 ° C, pure carbamide peroxide in particular should not be heated above 60 ° C.
Applications
Like hydrogen peroxide, carbamide peroxide is also used as a bleaching agent , e.g. B. for bleaching hair, for bleaching teeth or for fixing hair in permanent waves . The hydrogen peroxide-urea adduct is less active than liquid hydrogen peroxide, but more effective as a bleaching agent because it does not have to be made as alkaline as H 2 O 2 .
Carbamide peroxide is also suitable as a disinfectant , e.g. B. to reduce germs on contact lens surfaces or as an antiseptic for mouthwashes , ear drops or for superficial wounds and ulcers.
During deodorization , the strong oxidative effect of the hydrogen peroxide-urea adduct, e.g. B. exploited against thiols and amines .
Carbamide peroxide has proven to be a stable, easy-to-use, effective and, through suitable choice of reaction conditions, easily controllable oxidizing agent, which is particularly effective in the presence of organic catalysts, such as. B. maleic anhydride or inorganic catalysts, such as. B. Sodium tungstate is environmentally friendly and often provides the corresponding oxidation products in high yields.
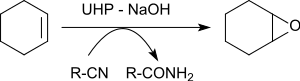
Thus, thiols become selectively disulfides , secondary alcohols become ketones , sulfides become sulfoxides and sulfones , nitriles become amides , N -heterocycles become amine oxides ,
aromatic hydroxyaldehydes to dihydric phenols ( Dakin reaction ) and, under suitable conditions, to the corresponding benzoic acids ,
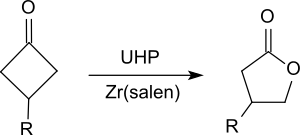
and ketones to esters , especially cyclic ketones, such as. B. substituted cyclohexanones or cyclobutanones to lactones ( Baeyer-Villiger oxidation ) oxidized.
The epoxidation of various alkenes in the presence of benzonitrile gives oxiranes in yields of 79 to 96%.
The oxygen atom transferred to the alkene comes from the peroxoimidic acid formed as an intermediate from benzonitrile . The resulting imidic acid tautomerizes to benzamide .
Individual evidence
- ↑ Entry on UREA PEROXIDE in the CosIng database of the EU Commission, accessed on May 22, 2020.
- ↑ a b c d data sheet hydrogen peroxide-urea from AlfaAesar, accessed on May 10, 2016 ( PDF )(JavaScript required) .
- ↑ a b c d data sheet Urea hydrogen peroxide from Sigma-Aldrich , accessed on May 10, 2016 ( PDF ).
- ↑ a b c d e f Data sheet hydrogen peroxide-urea for synthesis (PDF) from Merck , accessed on May 10, 2016.
- ↑ a b c H. Heaney, F. Cardona, A. Goti, AL Frederick: Hydrogen Peroxide-Urea . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2013, doi : 10.1002 / 047084289X.rh047.pub3 .
- ↑ a b C.-S. Lu, EW Hughes, PA Giguère: The crystal structure of the urea-hydrogen peroxide addition compound CO (NH 2 ) 2 H 2 O 2 . In: J. Am. Chem. Soc. tape 63 , no. 6 , 1941, pp. 1507-1513 , doi : 10.1021 / ja01851a007 .
- ↑ a b B. Karami, M. Montazerozohori, MH Habibi: Urea-Hydrogen Peroxide (UHP) oxidation of thiols to the corresponding disulfides promoted by maleic anhydride as mediator . In: molecules . tape 10 , no. 10 , 2005, pp. 1358-1363 , doi : 10.3390 / 10101385 ( mdpi.org [PDF]).
- ↑ a b M. Lukasiewicz, D. Bogdal, J. Pielichowski: Microwave-assisted oxidation of alcohols using urea hydrogen peroxide. In: 8th International Electronic Conference on Synthetic Organic Chemistry. ECSOC-8. Retrieved May 10, 2016 .
- ↑ a b c d R.S. Varma, KP Naicker: The Urea-Hydrogen Peroxide Complex: Solid-State Oxidative Protocols for Hydroxylated Aldehydes and Ketones (Dakin Reaction), Nitriles, Sulfides, and Nitrogen Heterocycles . In: Org. Lett. tape 1 , no. 2 , 1999, p. 189-191 , doi : 10.1021 / ol990522n .
- ↑ Patent WO2012069948A1 : 4- (5-Cyano-pyrazol-1-yl) -piperidine derivatives as GPR 119 modulators. Filed on November 9, 2011 , published on 31 May 2012 , Applicant: Pfizer Inc., inventors V. Mascitti, KF McClure, MJ Munchhof, RP Robinson, Jr
- ↑ D. Rong, VA Phillips, RS Rubio, MA Castro, RT Wheelhouse: A safe, convenient and efficient method for the preparation of heterocyclic N-oxides using urea-hydrogen peroxide . In: Tetrahedron Lett. tape 49 , no. 48 , 2008, p. 6933-6935 , doi : 10.1016 / tetlet.2008.09.124 .
- ↑ a b H. Heaney, AJ Newbold: The oxidation of aromatic aldehydes by magnesium monoperoxyphthalate and urea-hydrogen peroxide . In: Tetrahedron Lett. tape 42 , no. 37 , 2001, p. 6607-6609 , doi : 10.1016 / S0040-4039 (01) 01332-6 .
- ^ MY Rios, E. Salazar, HF Olivo: Baeyer-Villiger oxidation of substituted cyclohexanones via lipase-mediated perhydrolysis utilizing urea-hydrogen peroxide in ethyl acetate . In: Green Chem. Band 9 , 2007, p. 459-462 , doi : 10.1039 / B618175A .
- ^ A. Watanabe, T. Uchida, K. Ito, T. Katsuki: Highly enantioselective Baeyer-Villiger oxidation using Zr (salen) complex as catalyst . In: Tetrahedron Lett. tape 43 , no. 25 , 2002, pp. 4481-4485 , doi : 10.1016 / S0040-4039 (02) 00831-6 .
- ↑ L. Ji, Y.-N. Wang, C. Qian, X.-Z. Chen: Nitrile-promoted alkene epoxidation with urea-hydrogen peroxide (UHP) . In: Synth. Commun. tape 43 , no. 16 , 2013, p. 2256–2264 , doi : 10.1080 / 00397911.2012.699578 .