Maleic anhydride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Maleic anhydride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 2 O 3 | ||||||||||||||||||
| Brief description |
white scales with a pungent, pungent odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 98.06 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
0.93 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
53 ° C |
||||||||||||||||||
| boiling point |
202 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
|
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−469.8 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Maleic anhydride (according to IUPAC nomenclature: furan-2,5-dione , abbreviated very often as MSA ) is an organic-chemical compound from the group of carboxylic acid anhydrides , more precisely the anhydride of maleic acid , an unsaturated dicarboxylic acid . It is an intermediate product in the chemical industry .
Extraction and presentation
Historical procedure
Benzene oxidation
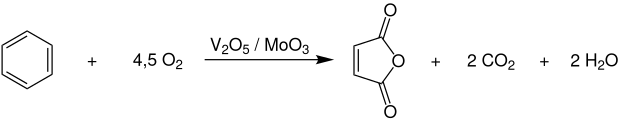
Until the beginning of the sixties, maleic anhydride was produced exclusively by the selective catalytic gas phase oxidation of benzene with atmospheric oxygen at temperatures of 400-450 ° C and pressures of 2-5 bar using vanadium pentoxide catalysts , which can also be modified with molybdenum trioxide or phosphoric acid.
The complete reaction is carried out in tube bundle reactors , in which the considerable heat of reaction (ΔH R = –1875 kJ · mol −1 ) is dissipated with the aid of molten salts and used to generate superheated high-pressure steam. The benzene conversion is 85-95%, but only a yield of 60-75% maleic anhydride is achieved. Another disadvantage is that about a third of the benzene is lost as carbon dioxide . Therefore, this process has become economically unattractive due to the high and continuously rising benzene prices and the emission limit values to be complied with .
Current procedures
Butene oxidation
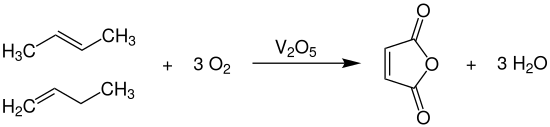
In response to this disadvantage, an improved process for the production of maleic anhydride was developed in the early 1960s. Accordingly, one starts from butadiene-free C4 fractions which essentially contain isobutene and the isomeric butenes (1-butene and 2-butene). This mixture is then reacted with atmospheric oxygen at temperatures of 350-450 ° C and pressures of 2-5 bar, also over vanadium pentoxide catalysts , which can be modified with oxides of titanium , molybdenum or antimony and are applied to supports with little surface area.
Here, too, tube bundle reactors are used , in which the considerable heat of reaction (ΔH R = –1315 kJ · mol −1 ) is dissipated with the aid of molten salts and used to generate superheated high-pressure steam. The selectivity to maleic anhydride is 45-60% based on the oxidizable butene content. The desired product is obtained after a two-stage distillation in low and high boiler columns in a purity of 99%. In addition to carbon dioxide and carbon monoxide , essential by-products are also acetic , acrylic , fumaric , crotonic and glyoxylic acid and formaldehyde .
Butane oxidation
With regard to the increasing demand for maleic anhydride, the more recent butene oxidation process also led to an unsatisfactory result due to the low selectivities. In view of this, Monsanto in the USA first developed an oxidation of butane as the cheapest feedstock for MSA in the mid-1970s . According to this process, n- butane is reacted with atmospheric oxygen at temperatures of 390-430 ° C. and a slight excess pressure over vanadyl pyrophosphate catalysts [(VO 2 ) 2 P 2 O 7 ] in the gas phase.
The reaction can be carried out analogously to the previous variants in tube bundle reactors and, more recently, also in fluidized bed reactors . The conversion of n- butane is 80-85%. This achieves a maleic anhydride yield of 50-60%, and in more recent process variants also 70-75%.
There is an analogy to the production of phthalic anhydride : While older processes start from naphthalene , modern processes use o- xylene .
properties
Physical Properties
Maleic acid forms colorless, needle-shaped crystals that melt at 52.8 ° C. The heat of fusion is 13.66 kJ mol −1 . The compound boils at 202 ° C. under normal pressure . The vapor pressure function results according to Antoine according to log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 3.79916, B = 1431.009 and C = −101.093 in the temperature range from 317 to 445 K. The heat of vaporization is 54.8 kJ · mol −1 .
Chemical properties
Maleic anhydride is very reactive. It hydrolyzes to maleic acid in water . The hydrolysis is exothermic at −34.9 kJ mol −1 . The corresponding monoesters or diesters are formed with alcohols . The reaction with ammonia or amines leads to the corresponding monoamides, which can be converted into the cyclic imides with elimination of water . As is typical for unsaturated compounds, maleic anhydride easily enters into addition reactions at the double bond . The corresponding dihalosuccinic acids , mono- or dihalomaleic acids or their anhydrides can be obtained with halogens . A hydrogenation may vary depending on the reaction conditions for succinic anhydride , 1,4-butanediol , tetrahydrofuran or butyrolactone lead. In Diels-Alder reactions it appears as a reactive dienophile . The reaction with 1,3-butadiene leads to 1,3,3a, 4,7,7a-hexahydro-2-benzofuran-1,3-dione (tetrahydrophthalic anhydride). This reaction is strongly exothermic by −263.6 kJ mol −1 .
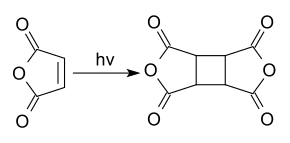
Photochemically, the dimerization to cyclobutanetetracarboxylic acid dianhydride takes place on exposure to UV light .
The compound can also be implemented in homo- or copolymerizations. The heat of polymerization is −59 kJ mol −1 .
Safety-related parameters
Maleic anhydride forms flammable vapor-air mixtures at higher temperatures. The compound has a flash point of 103 ° C. The explosion range is between 1.4% by volume (57 g / m 3 ) as the lower explosion limit (LEL) and 7.1% by volume (290 g / m 3 ) as the upper explosion limit (UEL). The lower explosion point is 91 ° C. The ignition temperature is 380 ° C. The substance therefore falls into temperature class T2.
use
Maleic anhydride is an industrial intermediate. It is mainly used for the production of unsaturated polyester resins and for the synthesis of surfactants , insecticides , herbicides , fungicides , growth regulators (e.g. maleic acid hydrazide ) and other chemical compounds. Part of it is reduced to 1,4-butanediol and tetrahydrofuran . With α-olefins , MA can be converted into strictly alternating copolymers , which are of great importance as lubricants .
Hazard assessment
Maleic anhydride was included in the EU's ongoing action plan ( CoRAP ) in 2013 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of maleic anhydride were concerns about high (aggregated) tonnage and high risk characterization ratio (RCR) as well as the suspected dangers of sensitizing properties. The re-evaluation took place from 2013 and was carried out by Austria . A final report was then published.
Web links
- International Chemical Safety Card (ICSC) for Maleic anhydride at the National Institute for Occupational Safety and Health (NIOSH).
Individual evidence
- ↑ a b c d e f g h i j k l m n o p Entry on maleic anhydride in the GESTIS substance database of the IFA , accessed on July 30, 2019 (JavaScript required)
- ↑ a b c d e f Entry on maleic anhydride. In: Römpp Online . Georg Thieme Verlag, accessed on April 8, 2014.
- ↑ Entry on Maleic anhydride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on July 30, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 108-31-6 or maleic anhydride ), accessed on September 17, 2019.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-25.
- ↑ a b c d e f Hans-Jürgen Arpe: Industrial organic chemistry - important preliminary and intermediate products . 6th edition. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim 2007, ISBN 978-3-527-31540-6 , p. 408 f .
- ↑ Manfred Baerns, Arno Behr, Axel Brehm, Jürgen Gmehling, Kai-Olaf Hinrichsen, Hanns Hofmann, Regina Palkovits, Ulfert Onken, Albert Renken: Technische Chemie . 2nd Edition. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany 2013, ISBN 978-3-527-33072-0 , p. 603 .
- ↑ a b c Brockhaus ABC Chemie , 3rd edition, FA Brockhausverlag Leipzig 1971, p. 836.
- ↑ a b c d e f g K. Lohbeck, H. Haferkorn, W. Fuhrmann, N. Fedke: Maleic and Fumaric Acids. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag, Weinheim, 2012, doi : 10.1002 / 14356007.a16_053 .
- ↑ Stull, DR: Vapor Pressure of Pure Substances Organic Compounds in Ind. Eng. Chem. 39 (1947) 517-540, doi : 10.1021 / ie50448a022 .
- ↑ Winstrom, LO; Kulp, L .: Vapor Pressure of Maleic Anhydride - Temperature Range from 35 ° to 77 ° in Ind. Eng. Chem. 41 (1949) 2584-2586, doi : 10.1021 / ie50479a044 .
- ↑ Conn, JB; Kistiakowsky, GB; Roberts, RM; Smith, EA: Heats of organic reactions. XIII. Heats of hydrolysis of some acid anhydrides in J. Am. Chem. Soc. 64 (1942) 1747-1752, doi : 10.1021 / ja01260a001 .
- ↑ Ghitau, M .; Ciopec, M .; Pintea, O .: Study on Diels-Alder reaction for the synthesis of tetrahydrophthalic anhydride in Rev. Chim. (Bucharest) 34 (1983) 299-305.
- ↑ Horie, T .; Sumino, M .; Tanaka, T .; Matsushita, Y .; Ichimura, T .; Yoshida, JI: Photodimerization of Maleic Anhydride in a Microreactor Without Clogging in Org. Process Res. Dev. 14 (2010) 405-410, doi : 10.1021 / op900306z .
- ↑ Bandrup, J .; Immergut, EH; Grulke, EA: Polymer Handbook , 4th Edition, Wiley & Sons Ltd. 2003, ISBN 978-0-471-47936-9 , p. II / 370.
- ↑ a b c E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ^ European Chemicals Agency (ECHA): Substance Evaluation Report and Conclusion Document .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Maleic anhydride , accessed on March 26, 2019.