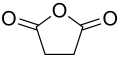
Succinic anhydride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Succinic anhydride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 4 O 3 | |||||||||||||||
| Brief description |
colorless, pungent smelling needles / crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 100.07 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.23 g cm −3 |
|||||||||||||||
| Melting point |
119 ° C |
|||||||||||||||
| boiling point |
261 ° C |
|||||||||||||||
| Vapor pressure |
1.2 h Pa (92 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−608.6 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Succinic anhydride is a heterocyclic organic chemical compound and the acid anhydride of succinic acid .
Extraction and presentation
Succinic anhydride can be obtained by reacting succinic acid and acetic anhydride , acetyl chloride or phosphorus oxychloride . Succinic anhydride is also formed when succinic acid is heated above 200 ° C. The compound can also be obtained by the catalytic hydrogenation of maleic anhydride .
properties
Physical Properties
Succinic anhydride is a white, flammable solid that melts at 119-120 ° C. The compound boils at 261 ° C. under normal pressure. The compound sublimes in a vacuum. According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 3.65221, B = 1433.874 and C = −144.107 in the temperature range from 365 K to 534 K. The solubility in water at 20 ° C is 67 g · l −1 . Slow hydrolysis takes place. The aqueous solution is acidic. The solubilities in aromatic hydrocarbons are relatively low. They amount in toluene 10 g · l -1 , in styrene 11 g · l -1 , in ethylbenzene 8 g · l -1 and in cumene 5 g · l -1 . The heat of formation of the solid is −608.6 kJ mol −1 . A value of −1537.1 kJ · mol −1 was determined for the heat of combustion .
Succinic anhydride vapor pressure function
Chemical properties
Succinic anhydride can be hydrolyzed to succinic acid in the presence of sodium hydroxide solution . A heat of reaction of −46.86 kJ mol −1 was determined in dioxane / water at 30 ° C. The reaction with alcohols gives the corresponding succinic acid half-esters. The compound can serve as a reagent for Friedel-Crafts acylations . The reaction with benzene produces the γ – oxocarboxylic acid β – benzoylpropionic acid. From Grignard compounds and succinic anhydride, γ-oxocarboxylic acid esters can also be obtained easily. The reaction with ammonia at higher temperatures gives the succinimide . The substance is flammable at higher temperatures, but it reacts very violently with oxidizing substances. The melt forms flammable vapor-air mixtures. The flash point is 157 ° C.
use
Succinic anhydride is used as a synthetic chemical for other chemical compounds, such as. B. in the multi-stage syntheses of 1-tetralone , 1-naphthol , tetralin and naphthalene as well as of N -chlorine- and N -bromo succinimide . The industrial production of citraconic anhydride (methyl maleic anhydride ) takes place by reacting succinic anhydride with formaldehyde . The compound is (formally) a starting compound for alkenylsuccinic anhydrides , which is used as a sizing agent in the paper industry . Its synthesis takes place via maleic anhydride and a long-chain alkene .
Health hazards
The consequences of touching or inhaling succinic anhydride are irritation of the skin and respiratory tract, which can cause inflammation of the mucous membranes. The compound was detected as a component in the main stream of a filterless cigarette.
Succinic anhydride was included in the EU's ongoing action plan ( CoRAP ) in 2013 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the intake of succinic anhydride were concerns about high (aggregated) tonnage and high risk characterization ratio (RCR) as well as the dangers arising from a possible assignment to the group of CMR substances and the suspected dangers of sensitizing properties. The re-evaluation took place from 2013 and was carried out by Austria . A final report was then published.
literature
- R. Kühn, V. Birett: Leaflets on dangerous working materials. Ecomed, Landsberg, 1986, Erg. Lfg.
- Beilstein 17
Individual evidence
- ↑ a b c d e f g h i j k l m Entry on succinic anhydride in the GESTIS substance database of the IFA , accessed on November 15, 2019(JavaScript required) .
- ↑ Data sheet succinic anhydride (PDF) from Merck , accessed on April 27, 2010.
- ↑ Entry on succinic anhydride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on November 15, 2019. Manufacturers and / or distributors can expand the harmonized classification and labeling .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-25.
- ↑ LF Fieser, EL Martin, RL Shriner, HC Struck: Succinic Anhydride In: Organic Syntheses . 12, 1932, p. 66, doi : 10.15227 / orgsyn.012.0066 ; Coll. Vol. 2, 1943, p. 560 ( PDF ).
- ^ A b Arnold Willmes: Pocket book chemical substances: elements - inorganics - organics - natural substances - polymers. 3rd, completely revised and exp. Edition. Frankfurt am Main 2007, ISBN 978-3-8171-1787-1 .
- ↑ a b K. Lohbeck, H. Haferkorn, W. Fuhrmann, N. Fedtke: Maleic and Fumaric Acids. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2005. doi : 10.1002 / 14356007.a16_053 .
- ^ A. Mclean, R. Adams: Succinic-α-d2, α'-d2, Acid and Its Derivatives. In: J. Am. Chem. Soc. 58, 1936, pp. 804-810. doi: 10.1021 / ja01296a038 .
- ↑ RC Weast, JG Grasselli, (Ed.): CRC Handbook of Data on Organic Compounds. 2nd Edition. CRC Press, Boca Raton, FL, 1989, p. 1.
- ^ DR Stull: Vapor Pressure of Pure Substances Organic Compounds. In: Ind. Eng. Chem. 39, 1947, pp. 517-540, doi: 10.1021 / ie50448a022 .
- ↑ PD Bartlett, K. Nozaki: The Polymerization of Allyl Compounds. III. The Peroxide-Induced Copolymerization of Allyl Acetate with Maleic Anhydride. In: J. Am. Chem. Soc. 68, 1946, pp. 1495-1504, doi: 10.1021 / ja01212a033 .
- ↑ a b Y. M. Yan, G. Pilcher: Enthalpies of combustion of succinic anhydride, glutaric anhydride, and glutarimide. In: J. Chem. Thermodyn. 22, 1990, pp. 893-898, doi: 10.1016 / 0021-9614 (90) 90177-R .
- ↑ JB Conn, GB Kistiakowsky, RM Roberts, EA Smith: Heats of organic reactions. XIII. Heats of hydrolysis of some acid anhydrides. In: J. Am. Chem. Soc. 64, 1942, pp. 1747-1752, doi: 10.1021 / ja01260a001 .
- ↑ J. Cason: β – Carbomethoxypropionyl Chloride In: Organic Syntheses . 25, 1945, p. 19, doi : 10.15227 / orgsyn.025.0019 ; Coll. Vol. 3, 1955, p. 169 ( PDF ).
- ↑ LF Somerville, CFH Allen: β-Benzoylpropionic Acid In: Organic Syntheses . 13, 1933, p. 12, doi : 10.15227 / orgsyn.013.0012 ; Coll. Vol. 2, 1943, p. 81 ( PDF ).
- ^ S. Hauptmann, J. Graefe, H. Remane : Textbook of organic chemistry. German publishing house for basic industry, Leipzig 1980, p. 421.
- ↑ a b S. Hauptmann, J. Graefe, H. Remane: Textbook of Organic Chemistry. German publishing house for basic industry, Leipzig 1980, p. 407.
- ↑ a b Römpp Chemie Lexikon. 9th edition. Georg Thieme Verlag, 1995.
- ^ European Chemicals Agency (ECHA): Substance Evaluation Report and Conclusion Document .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Succinic anhydride , accessed on March 26, 2019.



