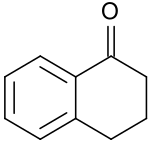
1 tetralone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1 tetralone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 10 O | |||||||||||||||
| Brief description |
clear light yellow to dark brown liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 146.19 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.099 g cm −3 (25 ° C ) |
|||||||||||||||
| Melting point |
2-7 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
2.7 Pa (20 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water, soluble in diethyl ether , benzene , toluene and p- xylene |
|||||||||||||||
| Refractive index |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
1-Tetralone is a bicyclic aromatic hydrocarbon with an α- keto group (benzocycloalkanone), which can also be understood as benzo-fused cyclohexanone and is used as a raw material for agrochemicals and pharmaceuticals.
Occurrence and representation
The basic body of the α-tetralons also occurs in natural substances, such as B. in the so-called Aristelegone A (4,7-dimethyl-6-methoxy-1-tetralone) from the Aristolochiaceae family used in traditional Chinese medicine .
1-tetralone by oxidation of tetralin
As early as 1933 by Heinrich Hock described, tends tetralin to autoxidation and forms, with oxygen in the air gradually 1- hydroperoxide . The heavy metal ion-catalyzed air oxidation of tetralin with Cr 3+ or Cu 2+ in the liquid phase leads via the hydroperoxide to a mixture of the intermediate stage 1-tetralol and the end product 1-tetralone.
Because the boiling points of the main component 1-tetralone (255–257 ° C) and the secondary component 1-tetralol (255 ° C) are practically identical, the latter is removed by chemical conversion.
1-tetralone by Friedel-Crafts reaction of 4-phenylbutyric acid
The starting compound 4-phenylbutyric acid (the sodium salt of which sodium phenylbutyrate is used to treat hyperammonaemia ) can be obtained from 3-benzoylpropionic acid via catalytic hydrogenation on a palladium contact with a yield of 96%. 3- Benzoylpropionic acid itself can be obtained from benzene and succinic anhydride in a yield of 77 to 82% by a Haworth reaction named after Robert Downs Haworth (a variant of the Friedel-Crafts reaction ) .
A more recent patent claims the synthesis of 4-phenylbutyric acid via Friedel-Crafts acylation of benzene and γ-butyrolactone with aluminum chloride at 60 ° C and working up with dilute sodium hydroxide solution and subsequent acidification in 94% crude yield and 81% pure yield.
The intramolecular cyclization of 4-phenylbutyric acid to 1-tetralone in 75 to 86% yield can be brought about by heating with polyphosphoric acid .
The acid-catalyzed cyclization can also be carried out with methanesulfonic acid . This reaction path with yields between 23 and 80% has been described as a working method for chemistry classes.
Also by adding catalytic amounts of strong Lewis acids , such as. B. the relatively easily accessible bismuth (III) bis (trifluoromethanesulfonyl) amide Bi (NTf 2 ) 3 , 4-phenylbutyric acid can be quantitatively converted into 1-tetralone at 180 ° C.
1-tetralone by Friedel-Crafts reaction of 4-phenylbutyric acid chloride
Significantly shorter reaction times than the Friedel-Crafts acylation with 4-phenylbutyric acid make it possible to use the acid chloride (by reaction with phosphorus pentachloride ) with superstoichiometric amounts of tin (IV) chloride SnCl 4 , with total yields of 85 to 91 according to method B of the specification % be achieved.
4-Phenylbutyric acid chlorides with electron donating groups ( electron donors ) can be cyclized to 1-tetralones under mild reaction conditions in yields greater than 90% in the solvent hexafluoroisopropanol HFIP, which forms strong hydrogen bonds .
1-tetralone by Friedel-Crafts reaction of γ-butyrolactone
The acylation of benzene with γ-butyrolactone with excess aluminum chloride by method A gives α-tetralone in yields of 91 to 96%.
The disadvantage of many variants of Friedel-Crafts acylation is the use of large amounts of AlCl 3 , polyphosphoric acid or PCl 5 for the preparation of the acid chlorides used, which cause considerable work-up costs and waste volumes.
The use of solid acidic catalysts based on zeolites and aluminosilicates for the reaction of benzene with γ-butyrolactone was also proposed, but no information was given on their efficiency. In this way is connected to the six-membered ring lactone δ-valerolactone the seven-membered ring-ketone 1-benzosuberone accessible.
properties
1-Tetralone is a clear, light yellow to dark brown liquid with a faint odor that is practically immiscible with water. Α-Tetralone is miscible with non-polar organic solvents.
Applications
1-Tetralone can be reduced to tetralin with lithium in liquid ammonia in a Birch reduction with 96% yield.
If the process management is changed and aqueous ammonium chloride solution is added after the ammonia has evaporated, the keto group is reduced to the secondary alcohol and 1-tetralol is obtained in 70% yield.
With calcium in liquid ammonia at −33 ° C, reduction to 1-tetralol takes place in 81% yield.
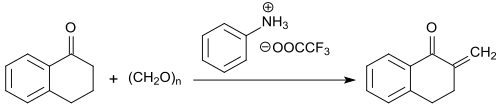
The methylene group α to the keto group is particularly reactive and is converted from formaldehyde in the form of trimeric paraldehyde in the presence of the trifluoroacetic acid salt of N-methylaniline to 2-methylene-1-tetralone with yields of up to 91%.
The 2-methylene ketone can be stored at temperatures below −5 ° C, but polymerizes completely within 12 hours at room temperature.
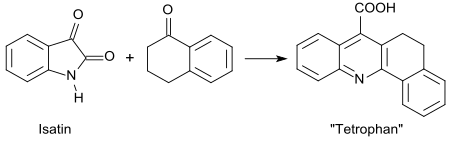
The Pfitzinger reaction of isatin with 1-tetralone produces the 3,4-dihydro-1,2-benzacridine-5-carboxylic acid known as tetrophane .
The reactivity of the α-methylene group is also made use of by the reaction of 1-tetralone with methanol at 270–290 ° C, in which 2-methyl-1-naphthol is formed in 66% yield with dehydration and formation of the aromatic naphthalene ring system .
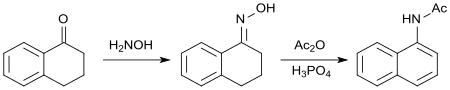
In the reaction of the oxime of 1-tetralone with acetic anhydride , with aromatization of the cycloalkanone ring, N- (1-naphthyl) acetamide is formed, which, like 1-naphthylacetic acid, acts as a synthetic auxin .
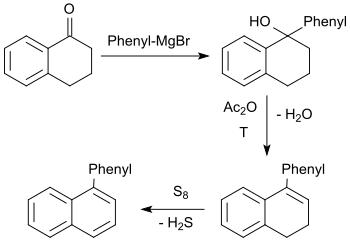
The tertiary alcohol formed in the Grignard reaction of 1-tetralone with phenylmagnesium bromide reacts with acetic anhydride with elimination of water to form 1-phenyl-3,4-dihydronaphthalene, which is dehydrated with elemental sulfur to 1-phenylnaphthalene in a total yield of approx. 45%.
The ruthenium (II) -catalyzed arylation of 1-tetralone using phenylboronic acid neopentylglycol ester gives 8-phenyl-1-tetralone in up to 86% yield.
1-Tetralone reacts with 5-aminotetrazole and an aromatic aldehyde in a multicomponent reaction under microwave radiation to form a four-membered heterocyclic ring system.
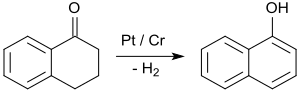
By far the most important application of 1-tetralone is in the synthesis of 1-naphthol by aromatizing dehydrogenation, e.g. B. on platinum catalysts at temperatures of 200 to 450 ° C.
1-naphthol is the starting material for the insecticide carbaryl and the beta blockers propranolol and nadolol , as well as for the antidepressant sertraline and the anti- protozoal therapeutic atovaquone .
The use of the 1-tetralons as a poison against clothes moths has not caught on on the market, despite its "hardly any unpleasant smell on the human sensory organs".
Individual evidence
- ↑ a b c d e Entry on α-Tetralone at TCI Europe, accessed on November 25, 2017.
- ↑ a b c d e f data sheet α-Tetralon at Sigma-Aldrich , accessed on November 25, 2017 ( PDF ).
- ^ A b William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, Boca Raton, FL, USA 2016, ISBN 978-1-4987-5429-3 , pp. 3-504 .
- ↑ a b c H.R. Snyder, FX Advertiser: α-Tetralone In: Organic Syntheses . 20, 1940, p. 94, doi : 10.15227 / orgsyn.020.0094 ; Coll. Vol. 3, 1955, p. 798 ( PDF ).
- ↑ a b c d C.E. Olson, AR Bader: α-Tetralone In: Organic Syntheses . 35, 1955, p. 95, doi : 10.15227 / orgsyn.035.0095 ; Coll. Vol. 4, 1963, p. 898 ( PDF ).
- ↑ a b D.-M. Cui, M. Kawamura, S. Shimada, T. Hayashi, M. Tanaka: Synthesis of 1-tetralones by intramolecular Friedel-Crafts reaction of 4-arylbutyric acids using Lewis acid catalysts . In: Tetrahedron Lett. tape 44 , no. 21 , 2003, p. 4007-4010 , doi : 10.1016 / S0040-4039 (03) 00855-4 .
- ↑ Data sheet 1-Tetralone, 97% from AlfaAesar, accessed on November 25, 2017 ( PDF )(JavaScript required) .
- ↑ P.-C. Kuo, Y.-C. Li, T.-S. Wu: Chemical constituents and pharmacology of the Aristolochia species . In: eJTCM . tape 2 , no. 4 , 2012, p. 249-266 , doi : 10.1016 / S2225-4110 (16) 30111-0 .
- ↑ H. Hock, W. Susemihl: Autoxidation of hydrocarbons: About a tetrahydro-naphthalene peroxide obtained by autoxidation (I. Commun.) . In: Ber. German Chem. Ges. Volume 66 , no. 1 , 1933, pp. 61-68 , doi : 10.1002 / cber.19330660113 .
- ↑ S. Bhattacharjee, Y.-R. Lee, W.-S. Ahn: Oxidation of tetraline to 1-tetralone over CrAPO-5 . In: Korean J. Chem. Eng. tape 34 , no. 3 , 2017, p. 701-705 , doi : 10.1007 / s11814-016-0310-4 .
- ↑ Patent US4473711 : Liquid-phase process for oxidation of tetralin. Applied on August 6, 1981 , published on September 25, 1984 , applicant: Union Carbide Corp., inventor: RW Coon.
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, Boca Raton, FL, USA 2016, ISBN 978-1-4987-5429-3 , pp. 3-502 .
- ↑ LF Somerville, CFH Allen: β-Benzoylpropionic acid In: Organic Syntheses . 13, 1933, p. 12, doi : 10.15227 / orgsyn.013.0012 ; Coll. Vol. 2, 1943, p. 81 ( PDF ).
- ↑ Patent US6372938B1 : Synthesis of 4-Phenylbutyric acid. Applied on May 21, 2001 , published on April 16, 2002 , applicant: SR Burzynski, inventor: SR Burzynski, L. Musial.
- ↑ V. Premasagar, VA Palaniswamy, EJ Eisenbraun: Methanesulfonic acid catalyzed cyclization of 3-arylpropanoic and 4-arylbutanoic acids to 1-indanones and 1-tetralones . In: J. Org. Chem. Band 46 , no. 14 , 1981, pp. 2974-2976 , doi : 10.1021 / jo00325a028 .
- ↑ MS Holden, RD Crouch, KA Barker: Formation of α-tetralone by intramolecular Friedel-Crafts acylation . In: J. Chem. Educ. tape 82 , no. 6 , 2005, p. 934-935 , doi : 10.1021 / ed082p934 .
- ↑ S. Antoniotti, E. Dunach: Facile preparation of metallic triflate and triflimidates by oxidative dissolution of metal powders . In: Chem. Commun. tape 8 , 2008, p. 993-995 , doi : 10.1039 / B717689A .
- ↑ H. Motiwala, RH Vekariya, J. Aubé: Intramolecular Friedel-Crafts acylation reaction promoted by 1,1,1,3,3,3-hexafluoro-2-propanol . In: Org. Lett. tape 17 , 2015, p. 5484-5487 , doi : 10.1021 / acs.orglett.5b02851 .
- ↑ SDK develops new catalyst for tetralone synthesis. In: Showa Denko KK News Release 2004. Showa Denko KK, accessed December 1, 2017 .
- ↑ a b Patent DE357063 : Process to protect fur and woolen fabrics against moths and other insects. Filed on October 25, 1921 , published on August 15, 1922 , Applicant: United chemical factories north Julius & Co ..
- ^ SS Hall, SD Lipsky, FJ McEnroe, AP Bartels: Lithium-ammonia reduction of aromatic ketones to aromatic hydrocarbons . In: J. Org. Chem. Band 38 , no. 18 , 1971, p. 2588-2591 , doi : 10.1021 / jo00817a004 .
- ↑ Z. Marcinow, PW Rabideau: Metal-ammonia reduction of α-tetralone. Competition between ring reduction, carbonyl reduction, and dimer formation . In: J. Org. Chem. Band 53 , no. 9 , 1988, pp. 2117-2119 , doi : 10.1021 / jo00244a054 .
- ↑ JR Hwu, YS Wein, Y.-J. Leu: Calcium metal in liquid ammonia for selective reduction of organic compounds . In: J. Org. Chem. Band 61 , no. 4 , 1996, pp. 1493-1499 , doi : 10.1021 / jo951219c .
- ↑ J.-L. Gras: Methylene ketones and aldehydes by simple, direct methylene transfer: 2-Methylene-1-oxo-1,2,3,4-tetrahydronaphthalene In: Organic Syntheses . 60, 1981, p. 88, doi : 10.15227 / orgsyn.060.0088 ; Coll. Vol. 7, 1990, p. 332 ( PDF ).
- ↑ I. Yuranov, L. Kiwi-Minsker, A. Renken: One-step vapor-phase synthesis of 2-methyl-1-naphthol from 1-tetralone . In: Appl. Catal. A . tape 226 , no. 1–2 , 2002, pp. 193-198 , doi : 10.1016 / S0926-860X (01) 00902-4 .
- ↑ MS Newman, WM Hung: An improved aromatization of α-tetralone oximes to N- (1-naphthyl) acetamides . In: J. Org. Chem. Band 38 , no. 23 , 1973, p. 4073-4074 , doi : 10.1021 / jo00987a029 .
- ↑ R. Weiss: 1-Phenylnaphthalene In: Organic Syntheses . 24, 1944, p. 84, doi : 10.15227 / orgsyn.024.0084 ; Coll. Vol. 3, 1955, p. 729 ( PDF ).
- ↑ K. Kitazawa, T. Kochi, F. Kakiuchi: Ruthenium-catalyzed arylation of ortho CH bond in an aromatic with an arylboronate: 8-Phenyl-1-tetralone In: Organic Syntheses . 87, 2009, pp. 209-217, doi : 10.15227 / orgsyn.087.0209 ; Coll. Vol. 11, 2009, pp. 1210-1216 ( PDF ).
- ↑ GP Kantin, M. Krasavin: Reaction of α-tetralone, 1 H -tetrazol-5-amine, and aromatic aldehydes upon microwave irradiation - a convenient method for the synthesis of 5,6,7,12-tetrahydrobenzo [h] tetrazolo [5,1-b] quinazolines . In: Chem. Heterocycl. Compd. tape 52 , no. 11 , 2016, p. 918-922 , doi : 10.1007 / s10593-017-1985-0 .
- ↑ Patent DE2421745 : Process for the production of α-naphthol by catalytic dehydrogenation of α-tetralone. Applied on May 6, 1974 , published on November 20, 1975 , Applicant: Sumitomo Chemical Co., Ltd., Inventor: K. Kudo, T. Ohmae, A. Uno.
- ↑ C. Kaiser, T. Jen, E. Garvey, WD Bowen, DF Colella, JR Wardell Jr .: Adrenergic agents. 4. Substituted phenoxypropanolamine derivatives as potential β-adrenergic agonists . In: J. Med. Chem. Volume 20 , no. 5 , 1977, pp. 687-689 , doi : 10.1021 / jm00215a014 .
- ↑ Patent DE2258995 : 2,3-cis-1,2,3,4-tetrahydro-5 [2-hydroxy-3- (tert-butylamino) propoxy] -2,3-naphthalenediol. Applied December 1, 1972 , published June 7, 1973 , Applicant: ER Squibb & Sons, Inc., Inventor: FR Hauck, CM Cimarusti, VL Narayan.
- ^ ME Condon et al .: Nondepressant β-adrenergic blocking agents. 1. Substituted 3-amino-1- (5,6,7,8-tetrahydro-1-naphthoxy) -2-propanols . In: J. Med. Chem. Volume 21 , no. 9 , 1978, p. 913-922 , doi : 10.1021 / jm00207a014 .
- ↑ K. Vukics, T. Fodor, J. Fischer, I. Fellevári, S. Lévai: Improved industrial synthesis of antidepressant sertraline . In: Org. Process Res. Dev. Band 6 , no. 1 , 2002, p. 82-85 , doi : 10.1021 / op0100549 .
- ^ BN Roy, GP Singh, PS Lathi, MK Agarwal: A novel process for synthesis of Atovaquone . In: Indian J. Chem. 52B, 2013, p. 1299-1312 ( res.in [PDF]).