Atovaquone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Atovaquone | ||||||||||||||||||
| other names |
2- [ trans -4- (4-chlorophenyl) cyclohexyl] -3-hydroxy-1,4-naphthoquinone ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 22 H 19 ClO 3 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 366.84 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
|
||||||||||||||||||
| Melting point |
222 ° C (polymorph III) |
||||||||||||||||||
| solubility |
practically insoluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Atovaquone is a drug used for the oral therapy of protozoal diseases such as malaria and toxoplasmosis as well as Pneumocystis pneumonia (PCP) caused by the fungus Pneumocystis jirovecii . Its mechanism of action is based on a structural analogy to ubiquinone (coenzyme Q) caused disruption of the nucleic acid synthesis of the pathogen.
Medical application
Atovaquon is offered by GlaxoSmithKline as a monotherapeutic agent under the brand name Wellvone for the treatment of mild to moderately severe forms of Pneumocystis pneumonia and in combination with Proguanil under the brand name Malarone for chemoprophylaxis and therapy of malaria.
The most common side effects observed were nausea, rash and itching, which were seen in more than 1 in 10 people; also diarrhea and vomiting, headache and insomnia.
Atovaquone is highly lipophilic , only slightly soluble in water and is almost completely bound to plasma proteins. The bioavailability is dose-dependent and shows great variability from patient to patient. When taken at the same time as a high-fat meal, bioavailability is two to three times higher than when it is taken on an empty stomach. The elimination half-life is 2 to 3 days. Excretion occurs largely unchanged (unmetabolized) with the stool.
Atovaquone is subject to a medical prescription .
synthesis
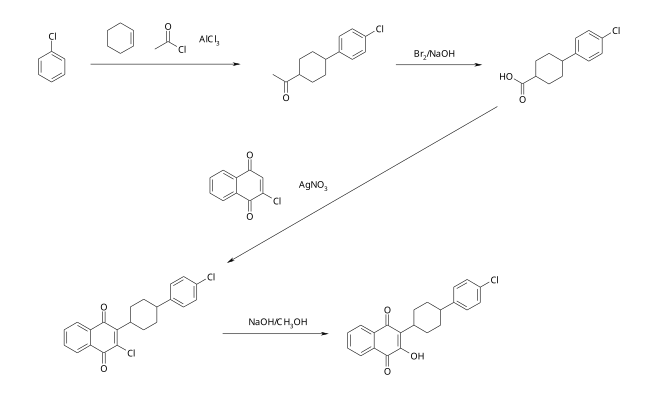
The synthesis of atovaquone starts with a Friedel-Crafts acylation of chlorobenzene using acetyl chloride in the presence of cyclohexene . The resulting chlorophenylcyclohexyl methyl ketone is oxidized with bromine to give the substituted cyclohexanecarboxylic acid. In the third step, there is an oxidative coupling with 2-chloro-1,4-naphthoquinone. The target molecule is obtained by the subsequent hydrolysis with methanolic sodium hydroxide solution.
properties
Atovaquone is a yellowish, crystalline solid that can come in three polymorphic forms. Form I shows an enantiotropic conversion to form III when heated to 197 ° C. Form II shows a similar behavior, which transforms enantiotropically into form III at 169 ° C. Form III shows no solid phase transformation up to the melting point at 222 ° C. The enthalpy of fusion is 35 kJ mol −1 . Forms I and II are monotropic to one another. Form I is the thermodynamically stable form at room temperature. All three forms can be obtained by means of solvent crystallization. Forms I and III both form a monoclinic crystal system; both crystallize in space group P 2 1 / c (space group no. 14) , but have different crystal structures. The mean inhibitory concentration ( IC 50 ) against Pneumocystis sp . is in vitro as well as in animal models, from 0.5 to 8 micrograms / ml.
Individual evidence
- ↑ a b c d e f Luciana Malpezzi, Claudio Fuganti, Elisabetta Maccaroni, Norberto Masciocchi, Antonio Nardi: Thermal and structural characterization of two polymorphs of Atovaquone and of its chloro derivative. In: Journal of Thermal Analysis and Calorimetry . Vol. 102, 2010, pp. 203-210, doi : 10.1007 / s10973-010-0685-0 .
- ↑ a b c Venkataswubramanian R. Tarur: Noval Polymorphs of Atovaquone and Process of , US Patent 2006/0241311 A1, October 26, 2006.
- ^ The Merck Index. An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition. Merck, Whitehouse Station NJ 2006, ISBN 0-911910-00-X , p. 865.
- ↑ a b data sheet Atovaquone from Sigma-Aldrich , accessed on May 9, 2017 ( PDF ).
- ↑ a b Specialist information Wellvone Suspension , GlaxoSmithKline, Munich. Status: October 2018.
- ^ Hermann Hager , Franz von Bruchhausen, Siegfried Ebel , Ulrike Holzgrabe: Hager's handbook of pharmaceutical practice. Follow-up work. Volume 4: Fabrics A - K. Springer, Berlin et al. 1998, ISBN 3-540-62644-1 , p. 122.