5-aminotetrazole
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | 5-aminotetrazole | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula |
|
||||||||||||
| Brief description |
white crystal powder |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 85.07 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
201-205 ° C |
||||||||||||
| solubility |
soluble in water (12 g · l −1 at 18 ° C) and in ethanol , insoluble in diethyl ether |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
5-aminotetrazole is a tetrazole- derived five-membered heterocyclic aromatic in which the H atom on the only carbon atom in the ring has been replaced by an amino group . The substance is an ampholyte , i. H. it has acidic and basic properties and is accessible to reactions at the acidic NH group in the 1-position and at the 5-amino group. Because of its extremely high nitrogen content of 82.3 percent by weight, 5-aminotetrazole is suitable as a gas generator in pyrotechnic applications , such as. B. in airbags and as a starting compound for explosives .
Occurrence and representation
The preparation of 5-aminotetrazole (then known as amidotetrazotic acid ) by the action of nitrous acid HNO 2 on aminoguanidine was described by Johannes Thiele in 1892 and the compound was patented as a starting material for azo dyes .
Even then it was found that when synthesized in aqueous media, 5-aminotetrazole crystallizes as a monohydrate “in leaves or prisms”, which changes into the anhydrous substance when heated. A structural formula for the so-called “amidotetrazotic acid” could not yet be given. The correct structural formula of Arthur Hantzsch published in 1901, the 5-aminotetrazole in the reaction of cyanamide and hydrogen azide received.
Instead of cyanamide, its dimer dicyandiamide can also be used. The use of sodium azide and concentrated hydrochloric acid avoids dealing with the extremely problematic hydrazoic acid , with 5-aminotetrazole being formed as a hydrate in 73% yield.
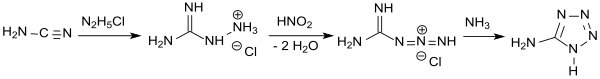
In a much more efficient and more controllable one-pot reaction, cyanamide is reacted with hydrazinium chloride to form aminoguanidinium chloride, which is diazotized to guanylazide hydrochloride with nitrous acid in acid, similar to the instructions given by J. Thiele . The mixture is then made weakly acidic with ammonia or sodium hydroxide and heated, cyclization taking place to give 5-aminotetrazole, which is obtained after drying as an anhydrous product in 74% yield.
The amination of 1 H -tetrazolium salts with hydroxylamine-O-sulfonic acid does not give 5-aminotetrazole, but rather a 2: 1 mixture of 1- and 2-aminotetrazole in a total yield of 38%.
In contrast to C-aminotetrazole 5-AT, N-aminotetrazoles, especially 2-aminotetrazole, tend to explode violently when heated quickly.
properties
Anhydrous 5-aminotetrazole is a white crystalline solid that dissolves in water and ethanol. The compound is not very toxic, temperature stable and not sensitive to impact. As an ampholyte , 5-AT reacts as a weak acid and against strong acids, such as. B. aqueous hydrogen halide solutions , nitric acid , perchloric acid , etc. as a weak base and forms the corresponding 5-aminotetrazolium salts.
Applications
5-aminotetrazole forms with alkali hydroxides and especially with basic amino compounds, such as. B. hydrazine , guanidine or aminoguanidine thermally and hydrolytically stable salts with extremely high nitrogen contents. The alkali salts are suitable as flame-coloring additives in fireworks , the ammonium salts as ionic liquids and as propellant additives .
The parent compound tetrazole can be obtained from 5-aminotetrazole by dediazonation , ie diazotization and subsequent reduction of the diazonium salt with hypophosphorous acid , in 77% yield.
Alkylation of 5-aminotetrazole, e.g. B. with dimethyl sulfate or methyl iodide leads to a mixture of predominantly 1-methyl-5-aminotetrazole in addition to 2-methyl-5-aminotetrazole.
If the 1-position is blocked by a substituent, the 5-position amino group is alkylated.
The acylation of 5-aminotetrazole with carboxylic acid anhydrides or carboxylic acid chlorides produces the corresponding 5-acylamidotetrazoles, which are suitable as foaming agents for polymer foams and for generating gas in airbags.
5-AT reacts with monochloroacetic acid to form 5-aminotetrazole-1-acetic acid in 53% yield, which can be nitrated with nitric acid to give the 5-nitroimino compound. The salts of 5-nitroiminotetrazole-1-acetic acid formed with nitrogen-rich cations are classified by the authors as environmentally friendly explosives.
The oxidation of 5-aryl- or 5-alkylaminotetrazoles opens up efficient access to isocyanides in high yields.
Benzyl isocyanide is obtained in this way in an overall yield of 67%.
5-aminotetrazole reacts with 1-tetralone and an aromatic aldehyde in a multicomponent reaction under microwave irradiation to form a four-membered heterocyclic ring system.
Acylation of 5-AT with methacryloyl chloride leads to the water-soluble monomer 5- (methacrylamido) tetrazole, which can be copolymerized with acrylamide and polymerized with N , N '-methylene bisacrylamide to form crosslinked gels with superabsorbent properties.
5-aminotetrazole is the starting compound for a number of so-called (high) energy density materials (HEDMs ), i. H. Explosives and propellants.
The most important application of 5-aminotetrazole is currently as an N 2 gas generator in airbags, since, in contrast to the sodium azide used previously, it is practically non-toxic, thermally stable and less sensitive to shock, and in contrast to the cheaper ammonium nitrate used by Takata as a propellant, it is not hygroscopic .
literature
- TM Klapötke: Chemistry of high-energy materials . de Gruyter, Berlin 2009, ISBN 978-3-11-020745-3 .
Individual evidence
- ↑ a b c Entry on 5-Amino-1H-tetrazole at TCI Europe, accessed on December 9, 2017.
- ↑ a b c data sheet 5-aminotetrazole from Sigma-Aldrich , accessed on December 9, 2017 ( PDF ).
- ↑ J. Thiele: Ueber Nitro- and Amidoguanidin . In: Justus Liebigs Ann. Chem. Band 270 , no. 1-2 , 1892, pp. 1-63 , doi : 10.1002 / jlac.18922700102 .
- ↑ Patent DE65584 : Process for the preparation of amidotetrazotic acid. Applied on September 20, 1891 , published on November 4, 1892 , applicant: J. Thiele, inventor: J. Thiele.
- ^ A. Hantzsch, A. Vagt: About the so-called Diazoguanidin . In: Justus Liebigs Ann. Chem. Band 314 , no. 3 , 1901, pp. 339-369 , doi : 10.1002 / jlac.19013140307 .
- ↑ Patent DE426343 : Process for the preparation of aminotetrazole. Registered on July 12, 1924 , published on March 6, 1926 , applicant: J. Thiele, inventor: R. Stollé, E. Schick.
- ↑ JS Minhina, RM Herbst: The reaction of nitriles with hydrazoic acid: Synthesis of monosubstituted tetrazoles . In: J. Org. Chem. Band 15 , no. 5 , 1950, pp. 1082-1092 , doi : 10.1021 / jo01151a027 .
- ↑ Patent US5424449 : Process for the preparation of 5-aminotetrazole. Filed October 28, 1994 , published June 13, 1995 , applicant: Olin Corp., inventor: EF Rothgery, KO Knollmueller.
- ↑ Patent US5594146 : Process for producing 5-aminotetrazole. Applied February 22, 1995 , published January 14, 1997 , Applicant: Nippon Carbide Kogyo KK, Inventors: M. Murotani, H. Mura, M. Taneka, H. Shibafuchi.
- ↑ R. Raap: Amination of tetrazole with hydroxylamine-O-sulfonic acid 1- and 2-aminotetrazoles . In: Can. J. Chem. Volume 47 , no. 19 , 1969, p. 3677-3681 , doi : 10.1139 / v69-606 .
- ↑ TM Klapötke, DG Piercey, J. Stierstorfer: Amination of energetic anions: high-performing energetic materials . In: Dalton Trans. Band 41 , no. 31 , 2012, p. 9451-9459 , doi : 10.1039 / C2DT30684K .
- ^ H. Gao, JM Shreeve: Azole-based energetic salts . In: Chem. Rev. Band 111 , no. 11 , 2011, p. 7377-7436 , doi : 10.1021 / cr200039c .
- ↑ G.-H. Tao, Y. Guo, Y.-H. Joo, B. Twamley, JM Shreeve: Energetic nitrogen-rich salts and ionic liquids: 5-aminotetrazole (AT) as a weak acid . In: J. Mater. Chem. Band 18 , 2008, p. 5524-5530 , doi : 10.1039 / b811506k .
- ^ RA Henry, WG Finnegan: An improved process for the deamination of 5-aminotetrazole . In: J. Am. Chem. Soc. tape 76 , no. 1 , 1954, p. 290-291 , doi : 10.1021 / ja01630a086 .
- ^ RA Henry, WG Finnegan: Mono-alkylation of sodium 5-aminotetrazole in aqueous media . In: J. Am. Chem. Soc. tape 76 , no. 3 , 1954, pp. 923-926 , doi : 10.1021 / ja01632a094 .
- ↑ RM Herbst, CW Roberts, EJ Harvill: The synthesis of 5-aminotetrazole derivatives . In: J. Org. Chem. Band 16 , no. 1 , 1951, p. 139-149 , doi : 10.1021 / jo01141a021 .
- ↑ Patent US5646292 : Blowing agents of tetrazoles and their derivatives. Applied on May 6, 1996 , published on July 8, 1997 , Applicants: Toyo Kasei Kogyo Co. Ltd., Inventors: S. Nakagawa, H. Ogawa, H. Tanaka, A. Onishi.
- ↑ F. Einberg: Alkylation of 5-substituiertem tetrazole with α-chlorocarbonyl compounds . In: J. Org. Chem. Band 35 , no. 11 , 1970, pp. 3978-3980 , doi : 10.1021 / jo00836a095 .
- ↑ Y.-H. Joo et al .: Energetic salts based on nitroiminotetrazole-containing acetic acid . In: J. Mater. Chem. Band 22 , no. 13 , 2012, p. 6123-6130 , doi : 10.1039 / C2JM30322A .
- ↑ G. Höfle, B. Lange: Oxidation of 5-aminotetrazoles: Benzyl isocyanide In: Organic Syntheses . 61, 1983, p. 14, doi : 10.15227 / orgsyn.061.0014 ; Coll. Vol. 7, 1990, p. 27 ( PDF ).
- ↑ GP Kantin, M. Krasavin: Reaction of α-tetralone, 1 H -tetrazol-5-amine, and aromatic aldehydes upon microwave irradiation - a convenient method for the synthesis of 5,6,7,12-tetrahydrobenzo [h] tetrazolo [5,1-b] quinazolines . In: Chem. Heterocycl. Compd. tape 52 , no. 11 , 2016, p. 918-922 , doi : 10.1007 / s10593-017-1985-0 .
- ^ A. Taden, AH Tait, A. Kraft: Synthesis and polymerization of 5- (methacrylamido) tetrazole, a water-soluble acidic monomer . In: J. Polym. Sci., Part A . tape 40 , no. 23 , 2002, p. 4333-4343 , doi : 10.1002 / pola.10509 .
- ↑ D. Fischer, TM Klapötke, DG Piercey, J. Stiersdorfer: Synthesis of 5-aminotetrazole-1 N -oxide and its azo derivative: A key step in the development of new energetic materials . In: Chemistry - A European Journal . tape 19 , no. 14 , 2013, p. 4602-4613 , doi : 10.1002 / chem . 201203493 .
- ↑ Patent EP0519485A1 : Propellant for gas generators. Registered on June 19, 1992 , published on December 23, 1992 , applicant: Dynamit Nobel AG, inventor: K. Redecker, W. Weuter.
- ↑ Patent US20090020197A1 : Gas generating compositions and airbag inflators. Filed July 16, 2007 , published January 22, 2009 , applicant: Key Safety Systems, Inc., inventor: EO Hosey.
- ↑ A Cheaper Airbag, and Takata's Road to a Deadly Crisis. The New York Times, August 26, 2016, accessed January 8, 2018 .