Aminoguanidine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Aminoguanidine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | CH 6 N 4 | |||||||||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 74.09 g mol −1 | |||||||||||||||||||||
| Melting point |
164 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Aminoguanidine is a chemical compound . It is a derivative of guanidine . The hydrochloride (CH 7 ClN 4 ) is usually found commercially .
Presentation and extraction
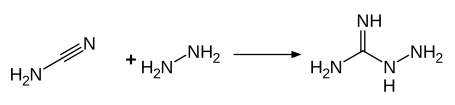
The industrial production of aminoguanidine takes place by reacting hydrazine with cyanamide in an aqueous solution.
The compound can also be obtained by reducing nitroguanidine using zinc in acetic acid.
properties
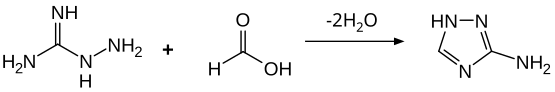
Aminoguanidine forms colorless crystals that are soluble in water and ethanol. As a basic compound, salts are formed with inorganic and organic acids. In the reaction with formic acid , cyclization to 3-amino-1,2,4-triazole takes place .
The compound reacts with nitrous acid in an acidic aqueous medium via the intermediate compound guanylazide to form 5-amino-1H-tetrazole . At neutral pH the reaction leads to tetrazene . The diazotization in glacial acetic acid yields 1,3-di- (tetrazolyl) -triazen .
use
Aminoguanidine is used as an intermediate in the manufacture of drugs , agrochemicals, and other chemical compounds (e.g. tetrazene , an explosive, and amitrole , a herbicide).
The compound is also being studied in medicine as it influences certain aging processes in the human body. It is used in the treatment of secondary damage from diabetes mellitus (inhibition of advanced glycation endproducts , NO synthase or glycosylation ).
Related links
- Aminoguanidinium hydrogen carbonate (C 2 H 8 N 4 O 3 )
- Aminoguanidinium hydrogen sulfate (CH 6 N 4 · H 2 SO 4 )
- Aminoguanidinium nitrate (CH 7 N 5 O 3 )
Individual evidence
- ↑ Entry on aminoguanidine. In: Römpp Online . Georg Thieme Verlag, accessed on September 26, 2018.
- ↑ Joubert, J .; van Dyk, S .; Malan, SF: Fluorescent polycyclic ligands for nitric oxide synthase (NOS) inhibition in Bioorg. Med. Chem. 16 (2008) 8952-8958, doi : 10.1016 / j.bmc.2008.08.049 .
- ↑ a b c Datasheet Aminoguanidine hydrochloride from Sigma-Aldrich , accessed on May 12, 2011 ( PDF ).
- ↑ External identifiers or database links for aminoguanidinium hydrochloride : CAS number: 1937-19-5, EC number: 217-707-7, ECHA InfoCard: 100.016.098 , PubChem : 2734687 , ChemSpider : 15211 , DrugBank : DB05383 , Wikidata : Q27095586 .
- ↑ a b c d e f T. Günther, B. Mertschenk, B. Schulz: Guanidine and Derivatives , in: Ullmanns Enzyklopädie der Technischen Chemie , Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2012; doi : 10.1002 / 14356007.a12_545.pub2 .
- ↑ Smith, GBL; Anzelmi, E .: Reduction of Nitroguanidines. III. Synthesis of aminoguanidines in J. Am. Chem. Soc. 57 (1935) 2730, doi : 10.1021 / ja01315a510 .
- ↑ Patinkin, SH; Horwitz, JP ; Lieber, E .: The Structure of Tetracene in J. Am. Chem. Soc. 77 (1955) 562-567, doi : 10.1021 / ja01608a014 .
- ↑ Reilly, J .; Teegan, MF; Carey, MF: in Sci. Proc. Roy. Dublin Soc. 24 (1948) 562.
- ↑ External identifiers or database links for aminoguanidinium hydrogen carbonate: CAS number: 2582-30-1, EC number: 219-956-7, ECHA InfoCard: 100.018.143 , GESTIS substance database : 570064 , PubChem : 164944 , ChemSpider : 144605 , Wikidata : Q27293343 .
- ↑ External identifiers of or database links for aminoguanidinium hydrogen sulfate, di (carbazamidine) sulfate : CAS number: 996-19-0, EC number: 213-628-7, ECHA InfoCard: 100.012.389 , PubChem : 2734952 , ChemSpider : 2016693 , Wikidata : Q27287642 .
- ↑ External identifiers or database links for aminoguanidinium nitrate: CAS number: 10308-82-4, EC number: 233-682-5, ECHA InfoCard: 100.030.607 , PubChem : 165859 , ChemSpider : 145354 , Wikidata : Q72437504 .