Tetrazene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
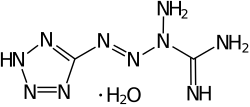
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetrazene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 6 N 10 • H 2 O | |||||||||||||||
| Brief description |
pale yellow, flaky crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 188.15 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.7 g cm −3 |
|||||||||||||||
| Melting point |
137–139 ° C (decomposition from 110 ° C) |
|||||||||||||||
| solubility |
almost insoluble in water, ethanol , diethyl ether , benzene and tetrachloromethane |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tetrazene is an energetic, solid nitrogen compound which, when ignited, evaporates, producing black smoke. Tetrazene is an important component of puncture-, impact- and heat-sensitive ignition charges, which are used in primers and electrical bridge ignitors for airbags .
properties
Tetrazene forms colorless to pale yellow, flaky crystals and can occur in various polymorphic forms. It exists in the crystalline state as a hydrated zwitterion. The compound is explosive and is assigned to the group of initial explosives. Tetrazene is long-term stable up to about 75 ° C and evaporates after a few seconds at 140 ° C. Dry tetrazene is very flammable and has a high explosion energy. The lead block bulge is 155 ml / 10 g. The compound is 1.0 J sensitive to impact or with 8 N friction sensitive . Tetrazene is practically insoluble in water , alcohol , ether , benzene and carbon tetrachloride . It is decomposed by boiling water or aqueous alkalis. The solubility in formic acid / acetic acid / acetic anhydride is good. Tetrazene dissolves in concentrated hydrochloric acid and in cold concentrated nitric acid (0–5 ° C) without decomposition and is excreted by dilution with water. Despite the low thermal stability, tetrazene samples stored under normal conditions for 8 years still show a purity of 99.9%.
Tetrazene is extremely sensitive to electrostatic discharges and in terms of mechanical sensitivity it is comparable to mercury (II) fulminate .
presentation
Tetrazene can be prepared ( synthesized ) by the reaction of sodium nitrite with a soluble salt of aminoguanidine in acetic acid .
use
Tetrazene is used as a high-energy sensitizer in primers based on lead typhnate ; the addition of tetrazene increases the sensitivity and reliability. Adding 5% tetrazene to lead azide makes it very sensitive to sting and impact. Tetrazene alone is a bad initial explosive , which loses its sensitivity if it is compressed too much. H. Tetrazene can easily be crushed to death .
history
Tetrazene was first made in 1910 by Hoffmann and Roth by treating aminoguanidine with nitrous acid .
Law
The manufacture and processing of tetrazene without permission is prohibited under the Explosives Act.
Substance group
Tetrazene should not be confused with the group of substances of the related tetrazenes , especially not with 1-tetrazene , as a derivative of which it can be understood.
Likelihood of confusion
Tetrazene can very easily be confused with tetrazene , tetrazine , tetrazole and tetracene .
Allergenicity
It is known from occupational health studies that exposure to tetrazene can trigger asthma , rhinitis and dermatitis .
literature
- Robert Matyas, Jiri Pachman: Primary Explosives . Springer, 2013, ISBN 978-3-642-28435-9 , pp. 189-194.
Individual evidence
- ↑ a b c d e f Entry on tetrazene. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b c d e f J. Köhler, R. Meyer, A. Homburg: Explosivstoffe. 10th, completely revised edition. Wiley-VCH, 2008, ISBN 978-3-527-32009-7 .
- ↑ There is not yet a harmonized classification for this substance . A labeling of 1- (5-tetrazolyl) -4-guanyl tetrazene hydrate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on July 19, 2019, is reproduced from a self-classification by the distributor .
- ↑ T. Guenther, B. Mertschenk, B. Schulz: guanidines and Derivatives , in: Ullmann's Encyclopedia of Industrial Chemistry , Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2012; doi : 10.1002 / 14356007.a12_545.pub2 .
- ^ KA Hoffmann, R. Roth: Diazo compounds from amidoguanidine, contributions to the knowledge of the diazohydrazo compounds (tetrazenes). In: Reports of the German Chemical Society. 43, 1919, pp. 1087-1095, doi: 10.1002 / cber.191004301187 .
- ^ PS Burge, M. Hendy, ES Hodgson: Occupational asthma, rhinitis, and dermatitis due to tetrazene in a detonator manufacturer. In: Thorax. 39 (6), Jun 1984, pp. 470-471. PMID 6235620 .

