Tetrazole
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Structural formula of the tautomer 1 H -tetrazole | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetrazole | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | CH 2 N 4 | |||||||||||||||
| Brief description |
colorless, sublimable leaflets (1 H -tetrazole) |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 70.06 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.406 g cm −3 |
|||||||||||||||
| Melting point |
157 ° C (1 H -tetrazole) |
|||||||||||||||
| pK s value |
4.89 |
|||||||||||||||
| solubility | ||||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tetrazole is a heterocyclic chemical compound for which three isomeric structures can be formulated.
Tautomerism
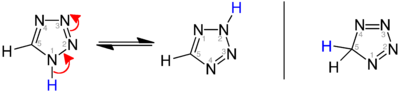
Depending on the position of the double bonds, a distinction is made between the isomeric structures 1 H -, 2 H - and 5 H -tetrazole. 1 H - and 2 H -etrazole form a tautomeric equilibrium which, in the crystalline solid, is on the side of the 1 H -etrazole. The 2 H tautomer dominates in the gas phase . Both the 1 H -etrazole (left) and the 2 H -etrazole (middle) can be understood as 6π-heteroaromatics:
presentation
1 H -tetrazol formed by the reaction of hydrocyanic acid with hydrazoic acid . This reaction is a 1,3-dipolar cycloaddition . The compound can also be obtained by the deamination of commercially available 5-aminotetrazole , which can easily be prepared from aminoguanidine .
properties
1 H -Tetrazole is a crystalline solid that occurs in two polymorphic forms. The two crystal forms are enantiotropic to one another. Form II is present below the transition temperature of −31 ° C. Form I is the thermodynamically stable crystal form above the transition temperature. This shows a melting point of 157 ° C. The enthalpy of fusion is 18.4 kJ mol −1 , the enthalpy of transformation at the solid-solid phase transition is 14.0 J mol −1 . According to August, the vapor pressure function results according to ln (P) = −A / T + B (P in Pa, T in K) with A = 10560 ± 168 and B = 31.148 ± 0.458 in the temperature range from 333 K to 404 K. From the A molar enthalpy of sublimation of 87.8 kJ · mol −1 can be derived from the vapor pressure function . The compound is having a standard enthalpy of formation of Δ f H solid = 236 kJ · mol -1 and Δ f H gas = 320 kJ · mol -1 strongly endothermic. The standard enthalpy of combustion Δ c H solid is −915.5 kJ mol −1 . The aqueous solution of 1 H -tetrazole is weakly acidic and has about the same acidity as acetic acid. A number of salts such as the lithium, sodium, potassium, rubidium, cesium, strontium, ammonium and hydrazinium salt could be produced and characterized. With an impact energy <4 J, the connection is extremely sensitive to impact . Opposite friction was found no sensitivity to 360 N.
meaning
Of the derivatives of 1 H -tetrazole, tetrazolium salts are of particular importance in biochemistry. The importance of the tetrazoles in pharmacy lies in the bioisosterism of the carboxy group . 1 H -tetrazoles substituted in the 5-position have similar physical properties to their carboxy analogues, but show a higher stability towards metabolism . Well-known pharmaceuticals are, for example, losartan or pentamethylene tetrazole or pentetrazole , an analeptic .
safety
1 H -Tetrazole is classified by the Federal Institute for Materials Research and Testing (BAM) as an explosive substance in substance group A within the meaning of the Explosives Act.
Web links
Individual evidence
- ↑ a b c d e Brockhaus ABC Chemie, VEB FA Brockhaus Verlag Leipzig 1965, p. 1391.
- ^ WC McCrone, D. Grabar, E. Lieber: Crystallographic Data. 42. Tetrazoles in Anal. Chem. 23 (1951) 543, doi : 10.1021 / ac60051a052 .
- ↑ a b c d e G. J. Kabo, AA Kozyro, AP Krasulin, VM Sevruk, LS Ivashkevich: Thermodynamic properties and tautomerism of tetrazole in J. Chem. Thermodyn. 25 (1993) 485-493, doi : 10.1006 / jcht.1993.1156 .
- ^ A b E. Lieber, SH Patinkin, HH Tao: The Comparative Acidic Properties of Some 5-Substituted Tetrazoles in J. Am. Chem. Soc. 73 (1951) 1792-1795, doi : 10.1021 / ja01148a111 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Goddard, R .; Heinemann, O .; Kruger, C .: α-1H-1,2,3,4-Tetrazole in Acta Cryst. C 53 (1997) 590-592, doi : 10.1107 / S0108270197000772 .
- ↑ a b Kiselev, VG; Cheblakov, PB; Gritsan, NP: Tautomerism and Thermal Decomposition of Tetrazole: High-Level from Initio Study in J. Phys. Chem. A 115 (2011) 1743-1753, doi : 10.1021 / jp112374t .
- ↑ van der Putten, N .; Heijdenrijk, D .; Schenk, H .: in Cryst. Struct. Comm. 1974, 321.
- ^ Wong, MW; Leung-Toung, R .; Wentrup, C .: Tautomeric Equilibrium and Hydrogen Shifts of Tetrazole in the Gas Phase and in Solution in J. Am. Chem. Soc. 115 (1993) 2465-2472.
- ↑ Razynska, A .; Tempczyk, A .; Malinski, E .; Szafranek, J .; Grzonka, Z .; Hermann, P .: in J. Chem. Soc. Perkin Trans. 2 1983, 379.
- ^ RA Henry, WG Finnegan: An Improved Procedure for the Deamination of 5-Aminotetrazole in J. Am. Chem. Soc. 76 (1954) 290-291, doi : 10.1021 / ja01630a086 .
- ↑ F. Kurzer, LEA Godfrey: Syntheses of heterocyclic compounds from aminoguanidine in Angew. Chem. 75 (1963) 1157-1175, doi : 10.1002 / anie.19630752303 .
- ^ FR Hilgeman, FYN Mouroux, D. Mok, MK Holan: Phase Diagrams of Binary Solid Azole Systems in J. Chem. Eng. Data 34 (1989) 220-222, doi : 10.1021 / je00056a022 .
- ↑ a b A.A. Balepin, VP Lebedev, EA Miroshnichenko, GI Koldobskii, VA Ostovskii, BP Larionov, BV Gidaspov, Yu.A. Lebedev: Energy effects in polyphenylenes and phenyltetrazoles in Svoistva Veshchestv Str. Mol., 1977, 93-98.
- ↑ a b W.S. McEwan, WS, MW Rigg: The heats of combustion of compounds containing the tetrazole ring in J. Am. Chem. Soc. 73 (1951) 4725-4727, doi : 10.1021 / ja01154a072 .
- ↑ a b Klapötke, TM; Stein, M .; Stierstorfer, J .: Salts of 1H-Tetrazole - Synthesis, Characterization and Properties in Z. Anorg. General Chem. 634 (2008) 1711-1723, doi : 10.1002 / zaac.200800139 .
- ↑ Announcement of the new findings made by BAM since 1987 in accordance with Section 2 SprengG - notification of assessment no. 301 of March 31, 1994 pdf link .