Amitrole
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Amitrole | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 2 H 4 N 4 | ||||||||||||||||||
| Brief description |
colorless, odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 84.08 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.14 g cm −3 |
||||||||||||||||||
| Melting point |
159 ° C |
||||||||||||||||||
| boiling point |
Decomposes on heating |
||||||||||||||||||
| solubility |
good in water (280 g l −1 at 25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
DFG / Switzerland: 0.2 mg m −3 (measured as inhalable dust ) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
76.8 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Amitrol is a chemical compound from the triazole group that is used as a herbicide .
Extraction and presentation
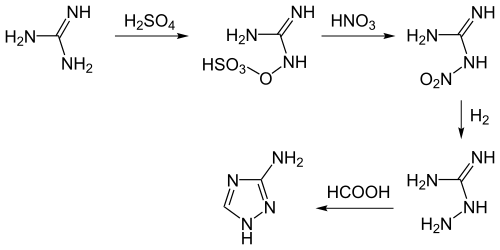
Amitrole is obtained from guanidine . This reacts with sulfuric acid to form guanidine sulfate and further with nitric acid to form nitroguanidine . The nitroguanidine is hydrogenated to aminoguanidine and cyclized to amitrole with formic acid.
history
In the USA , Amitrol triggered the “ cranberry scare ” of 1959. The herbicide was approved by the Department of Agriculture for use on cranberry fields in 1957, provided that it was only used after the harvest. Some farmers apparently did not adhere to this requirement, and the Food and Drug Administration (FDA) confiscated some lots of amitrole-contaminated cranberries in 1957. They were stored in cold stores until the dangerousness of the chemical was clarified. According to a long-term study completed in 1959, amitrol caused thyroid cancer in rats . A law passed in 1958 stipulated that food must not contain any traces of any carcinogenic substances found in animal experiments. The FDA therefore had the frozen berries destroyed. On November 9, 1959, a few weeks before Thanksgiving , Health Secretary Arthur S. Flemming recommended not buying cranberries until the FDA checked all inventory for Amitrol. Both the cranberry producers and the manufacturers of Amitrol ( American Cyanamid and Amchem ) protested violently, especially since no contaminated berries were found in the year in question.
The prices for cranberries nevertheless fell sharply, large supermarket chains stopped selling and some restaurants took the berries off their menus. To reassure the public, Agriculture Secretary Ezra Taft Benson announced that his family would be serving cranberries for Thanksgiving. Vice President Richard Nixon ate four servings of cranberries at dinner.
The FDA managed to check all stocks well in advance of Thanksgiving. So the excitement quickly subsided and the matter was forgotten. As a consequence of the “ cranberry scare ”, the American pesticide industry stepped up its public relations work .
use
Amitrol is used as a herbicide, especially against broad-leaved weeds. After treatment, the chlorophylls in the plants will fade .
Together with DCMU , Amitrol was part of the total herbicide Ustinex .
Mode of action
The mode of action of Amitrol has long been controversial. Today it is assumed that the synthesis of pigments, more precisely the lycopene cyclases in the carotenoid biosynthesis, is inhibited. In the past, there was also talk of inhibiting root growth or histidine biosynthesis.
Admission
In some EU countries, Amitrol is approved as a plant protection product , but not in Germany, Austria or Switzerland. The EU member states will revoke the approvals for plant protection products that contain amitrole as an active ingredient by September 30, 2016.
safety instructions
Amitrol is only slightly toxic, but is considered to be carcinogenic.
Individual evidence
- ↑ a b c d e f g h i Entry on Amitrol in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on Amitrole in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 61-82-5 or Amitrol ), accessed on November 2, 2015.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-22.
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 683 ( limited preview in Google Book search).
- ^ Thomas R. Dunlap: DDT: Scientists, Citizens and Public Policy . Princeton University Press, 1981, ISBN 0-691-04680-8 , pp. 107-108.
- ↑ Hans Steiner: Current Problems in Integrated Plant Protection: Lectures of the 2nd meeting of the working group "Integrated Plant Protection" of the German Phytomedical Society in Wilhelmsbad near Hanau November 6th - 7th, 1972 . Dr. Dietrich Steinkopff Verlag, 1975, ISBN 978-3-642-72311-7 , p. 27 ( Google Books ).
- ↑ Jasmina Muraja-Ljubičić, Mercedes Wrischer, Nikola Ljubešić: Influence of the Herbicides Amitrole and Norflurazon on Greening of Illuminated Potato Microtubers . In: Journal of Nature Research C . tape 54 , no. 5-6 , 1999, pp. 333-336 , doi : 10.1515 / znc-1999-5-607 ( PDF ).
- ↑ DR Heim, IM Larrinua: Primary Site of Action of Amitrole in Arabidopsis thaliana Involves Inhibition of Root Elongation but Not of Histidine or Pigment Biosynthesis. In: Plant Physiology . Volume 91, number 3, November 1989, pp. 1226-1231, PMC 1062144 (free full text) ( PDF ).
- ^ Inhibitors of histidine biosynthesis
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Amitrole (aminotriazole) in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 12, 2016.
- ↑ Implementing Regulation (EU) 2016/871 of the Commission of June 1, 2016 on the non-renewal of the approval of the active substance Amitrole according to Regulation (EC) No. 1107/2009 of the European Parliament and of the Council on the placing of plant protection products on the market and amending the Implementing Regulation ( EU) No. 540/2011 of the Commission .
Web links
- FAO assessment of amitroles (en) (PDF file; 172 kB)